Figures & data
Figure 1 MCF7 cancer cells induce the expression of MCT4 in cancer-associated fibroblasts. MCF7 cells were co-cultured with fibroblasts and then we observed the distribution of MCT4 (red) by fluorescence microscopy. Cultures of MCF7 cells alone or fibrobasts alone (monotypic cultures) were also processed in parallel. Note that MCF7 cells alone do not express significant amounts of MCT4. Similarly, fibroblasts alone do not express MCT4. However, when these two cell types are co-cultured, MCT4 is selectively upregulated in the cancer-associated fibroblasts. Epithelial cancer cells were visualized by keratin staining (green).
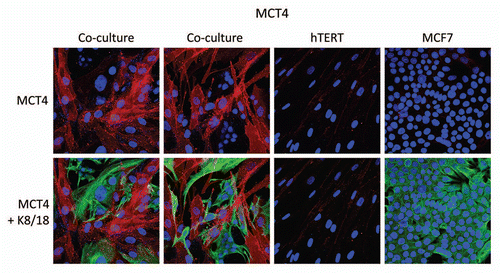
Figure 2 The induction of MCT4 in cancer-associated fibroblasts is due to oxidative stress and is prevented by antioxidants. MCF7 cells were co-cultured with fibroblasts and then we observed the distribution of MCT4 (red) by fluorescence microscopy. Since MCT4 expression is controlled by HIF1 and HIF1 is also activated by pseudo-hypoxia (oxidative stress), we assessed the effects of anti-oxidants on this process. Note that treatment with N-acetyl-cysteine (NAC; 10 mM), a powerful antioxidant, is sufficient to block that upregulation of MCT4 in cancer-associated fibroblasts, as predicted. Epithelial cancer cells were visualized by keratin staining (green).
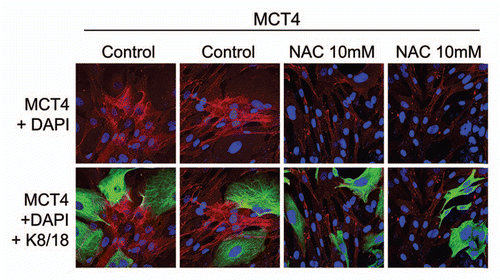
Figure 3 Fibroblasts induce the expression of MCT1 in MCF7 cancer cells. MCF7 cells were co-cultured with fibroblasts and then we observed the distribution of MCT1 (red) by fluorescence microscopy. Cultures of MCF7 cells alone or fibrobasts alone (monotypic cultures) were also processed in parallel. Note that MCT1 is not well expressed in MCF7 cells or fibroblasts, when cultured individually. However, under conditions of co-culture, MCT1 is specifically induced in MCF7 cells. Epithelial cancer cells were visualized by keratin staining (green).
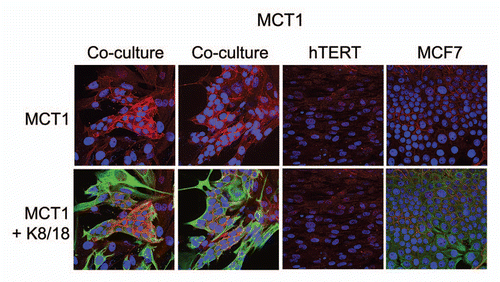
Figure 4 MDA-MB-231 cells also induce the expression of MCT4 in cancer-associated fibroblasts. MDA-MB-231 cells (expressing GFP) were co-cultured with fibroblasts and then we observed the distribution of MCT4 (red) by fluorescence microscopy. Cultures of MDA-MB-231 cells alone or fibrobasts alone (monotypic cultures) were also processed in parallel. Note that although MDA-MB-321 cells constitutively overexpress MCT4, they are also capable of inducing MCT4 expression in fibroblasts during co-culture (see also higher magnification insets). Epithelial cancer cells were visualized via GFP (green).
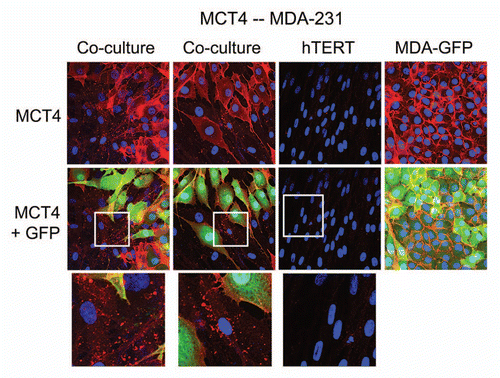
Figure 5 MCT4 is expressed in the fibroblastic stromal compartment of human breast cancers. Note that MCT4 staining is selectively localized to the fibroblastic tumor stromal compartment of human breast cancers. Two representative images are shown. Both clearly show that MCT4 staining is absent from the tumor epithelial cells, but is present in the surrounding stroma. Panel (A) shows DCIS-like lesions and the surrounding MCT4(+) tumor stroma. Panel (B) shows that MCT4 staining outlines the cancer-associated fibroblasts that surround nests of epithelial cancer cells. Original magnification, 40×.
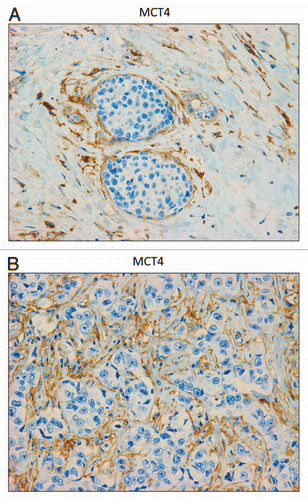
Figure 6 MCT1 is expressed in the epithelial compartment of human breast cancers. Note that only epithelial cancer cells express MCT1 in human breast tumor samples. Two representative images are shown. Both clearly show that MCT1 staining is present in the tumor epithelial cells, but is absent in the surrounding stroma. Panel (A) shows DCIS-like lesions and the surrounding MCT1(+) epithelial cancer cells. Panel (B) shows that MCT1 staining identifies the epithelial cancer cells within the “cancer cell nests.” The original magnifications for (A and B) are 40× and 60×, respectively.
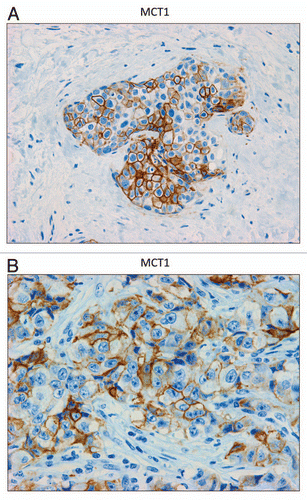
Figure 7 CD147 is expressed in the epithelial compartment of human breast cancers. Note that epithelial cancer cells express CD147 in human breast tumor samples. Two representative images are shown. Both clearly show that CD147 staining is present in the tumor epithelial cells, but is largely absent in the surrounding stroma. Panel (A) shows DCIS-like lesions and the surrounding CD147(+) epithelial cancer cells. Panel (B) shows that CD147 staining identifies the epithelial cancer cells within the “cancer cell nests.” The original magnifications for both (A and B) are 60×.
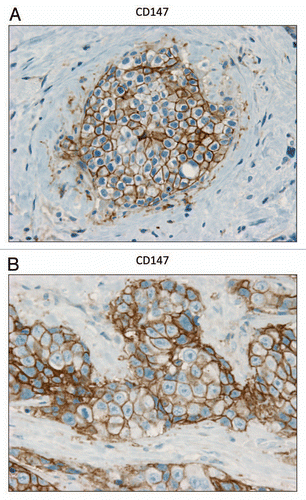
Figure 8 Informatics analysis of the transcriptional levels of MCT4 in human breast cancers. We used informatics analysis to determine whether the mRNA transcript for MCT4 is commonly upregulated in human breast cancer. (Left) Note that MCT4 (SLC16A3) is overexpressed in all types of breast cancer (relative to normal breast tissue), including both ER(+) and ER(−) cancer sub-types. (Right) In the HER2(+) sub-type, we observed an association with clinical outcome; increased MCT4 transcript levels were associated with decreased overall survival (N = 14 patients).
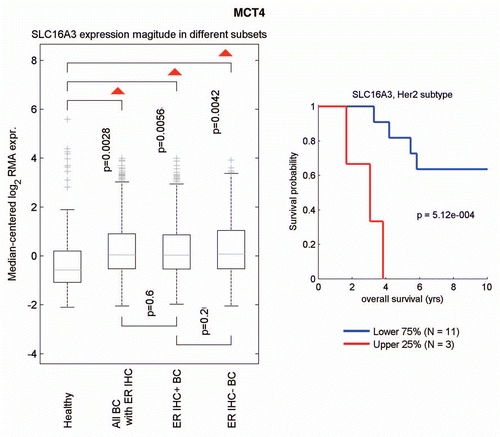
Figure 9 Overexpression of MCT4 in fibroblasts functionally protects both cancer cells and fibroblasts against cell death under co-culture conditions. To assess the possible functional consequences of MCT4 expression in fibroblasts, we generated an hTERT-fibroblast cell line stably overexpressing MCT4. Similarly, we also generated hTERT-fibroblast cell lines overexpressing MCT1, and the vector alone (Lv-105). Then, these three matched fibroblast cell lines were individually co-cultured with GFP-tagged MCF7 cells, and cell death in both fibroblasts and cancer cells was monitored by FACS analysis (See the Materials and Methods section). (A) Note that co-culture with MCT4-expressing fibroblasts protects MCF7 cells against cell death, by nearly 2-fold (p = 0.035). In contrast, the effects of MCT1-expressing fibroblasts on MCF7 cell death were not significant. (B) Note that co-cultured fibroblasts expressing MCT1 (p = 0.01) or MCT4 (p = 0.002) both showed >2-fold protection against cell death. (C) However, when MCT4 fibroblasts were cultured alone, in the absence of cancer cells, they showed a >2-fold increase in cell death (p = 0.005). Thus, expression of MCT4 in fibroblasts functionally prolongs the life of both cancer cells and fibroblasts, under co-culture conditions.
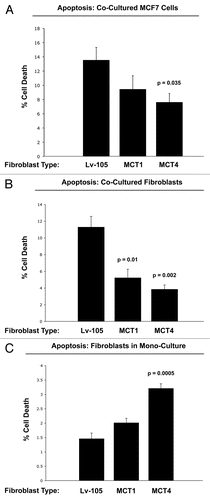
Figure 10 The lactate shuttle: an energy transfer mechanism in normal tissue and human cancers. MCT4 functions primarily as a transporter that extrudes lactate from cells that are undergoing aerobic glycolysis and lack functional mitochondria. Two normal physiological examples of this are fast-twitch fibers in skeletal muscle and astrocytes within the brain. After lactate is extruded by MCT4, the lactate is then taken up by other MCT transporters in adjacent cells, such as slow-twitch (mitochondrial-rich) fibers in muscle or neurons in the brain. To accomplish the scavenging of lactate, slow-twitch muscle fibers use MCT1, while neurons use MCT2. In the brain, this phenomenon has been referred to as “neuron-glia metabolic coupling,” while in skeletal muscle it is known as the “lactate shuttle.” Our current studies support the hypothesis that similar metabolic-coupling occurs between cancer-associated fibroblasts and adjacent tumor cells.
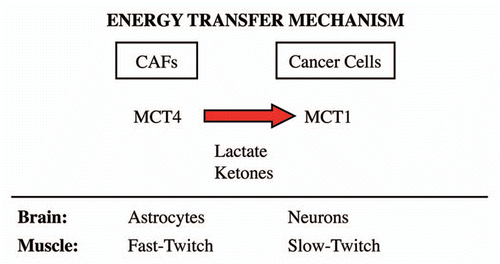
Table 1 Overexpression of MCT4 and other “Astrocyte Markers” in the tumor stroma of human breast cancers