Figures & data
Figure 1 Measuring the binding affinities of SH2 and PTB domains for a phosphopeptides derived from c-Mpl pY631 using protein microarrays. (A) Fluorescence images of SH2 and PTB domain microarrays in one well of a 96-well microtiter plate, obtained using a 633 nm and 543 nm lasers. The fluorescence in the 633 nm channel (red) arises from a trace amount of Cy5-labeled BSA that was added to each protein before arraying. The fluorescence in the 543 nm channel (green) arises from the 5,6-TAMRA-labeled phosphopeptide and represents a binding event between the peptide and the immobilized SH2 or PTB domains. Spots containing the SH2 domains of Tensin2 and NCK2 are highlighted with a white rectangle. (B) Saturation binding curves obtained by probing SH2/PT B domain microarrays with eight different concentrations of the phosphopeptides. Plots show fluorescence as a function of peptide concentration for the two highest intensity interactions on the arrays. The data were fit to Equationequation (1)(1)
(1) .
![Figure 1 Measuring the binding affinities of SH2 and PTB domains for a phosphopeptides derived from c-Mpl pY631 using protein microarrays. (A) Fluorescence images of SH2 and PTB domain microarrays in one well of a 96-well microtiter plate, obtained using a 633 nm and 543 nm lasers. The fluorescence in the 633 nm channel (red) arises from a trace amount of Cy5-labeled BSA that was added to each protein before arraying. The fluorescence in the 543 nm channel (green) arises from the 5,6-TAMRA-labeled phosphopeptide and represents a binding event between the peptide and the immobilized SH2 or PTB domains. Spots containing the SH2 domains of Tensin2 and NCK2 are highlighted with a white rectangle. (B) Saturation binding curves obtained by probing SH2/PT B domain microarrays with eight different concentrations of the phosphopeptides. Plots show fluorescence as a function of peptide concentration for the two highest intensity interactions on the arrays. The data were fit to Equationequation (1)(1) Fobs=F0+Fmax[pep]KD+[pep](1) .](/cms/asset/fe0cd3c6-551f-4f91-b4b4-5019d2c76020/kccy_a_10915776_f0001.gif)
Figure 2 Western blots of engineered BaF3 cells (2a) and pY631 peptide bead co-precipitation of Tensin2 (2b). (A) BaF3/c-Mpl cells were engineered to express Tensin2 fused with a c-Myc tag. (B) BaF3/c-Mpl/c-Myc-tagged Tensin2 cells were lysed and co-precipitated with biontinylated Neutravidin beads as control or with biotinylated pY631 oligo-peptide Neutravidin beads. Precipitated material was analyzed by western blotting to c-Myc tagged Tensin2 or STAT5.
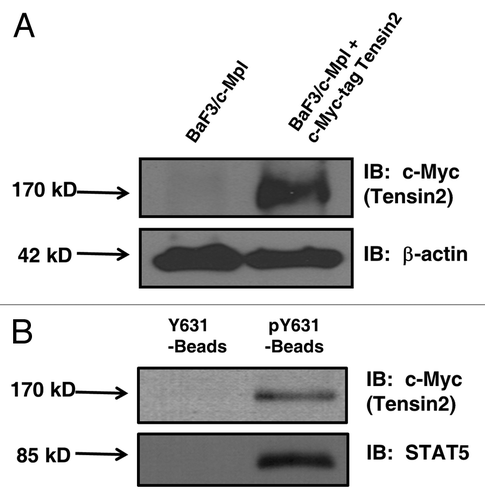
Figure 3 Tensin2 is phosphorylated in a TPO-dependent fashion. BaF3/c-Mpl/c-Myc-tagged Tensin2 cells were starved of serum and then stimulated with 100 ng/mL of TPO for various time points. Cells were then lysed and immune-purified with anti-c-Myc antibody or mouse IgG as control. Immune precipitates were analyzed by western blotting.
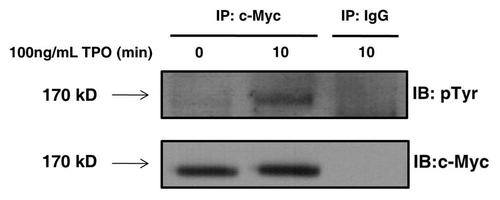
Figure 4 Tensin2 affects TPO-dependent cellular growth. (A) Graphic representation of downregulation of Tensin2 mRNA levels using siRNA specific to Tensin2. After ∼36 hr of treatment with Tensin2 specific mRNA or non-target siRNA as control, semi-quantitative PCR was performed on UT7-TPO cells using various numbers of PCR cycles. Semi-quantitative PCR of GAPDH mRNA was performed as RNA loading control. Densitometry was performed and graphically represented above after normalizing for loading. (B) MTT assay comparing the Tensin2 siRNA treated UT7-TPO cells versus negative control at various TPO concentrations grown for 40–48 hours. Tensin2 siRNA treated cells had a significant reduction in cellular proliferation at all doses of TPO.
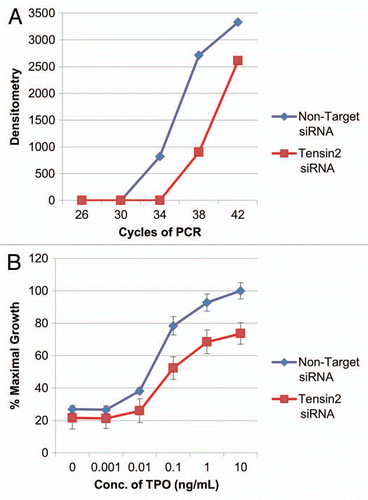
Figure 5 Tensin2 is involved in TPO-mediated activation of Akt. siRNA-treated UT7-TPO cells were starved of serum and then stimulated with 5 ng/mL (of TPO ) for 20 min. Cells were then lysed and analyzed by western blotting for pAkt and total Akt. TPO-mediated phosphorylation of Akt was substantially diminished in the Tensin2 knockdown cells.
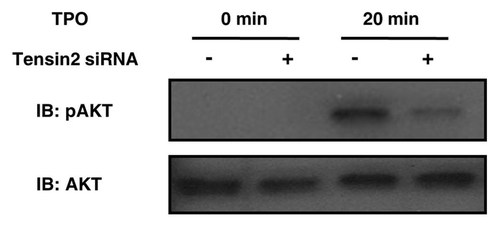
Figure 6 Tensin2 is phosphorylated at its PI3K consensus binding motif in a TPO-dependent fashion. BaF3/c-Mpl/c-Myc-tagged Tensin2 cells were starved of serum and then stimulated with 100 ng/mL of TPO for various time points. Cells were then lysed and immune-purified with anti-c-Myc antibody or mouse IgG as control. Immune precipitates were analyzed by western blotting.
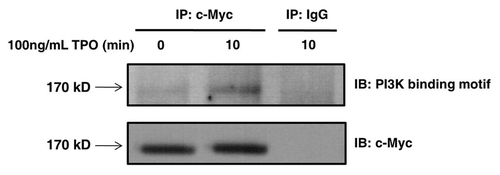
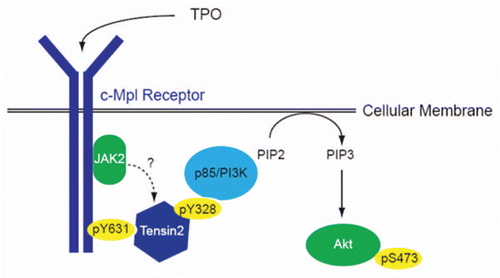
Figure 7 Proposed role of Tensin2 in TPO/c-Mpl signaling of PI3K/Akt. Upon binding of TPO to its receptor, c-Mpl, the intracellular domain of c-Mpl become phosphorylated, one site being at Y631. Tensin2 is then recruited to that site and is phosphorylated. Once phosphorylated, it may act to recruit the p85 subunit of PI3K, which then goes on to activate Akt and affects downstream functions including cell growth.