Figures & data
Figure 1 DNA single-strand break repair. Resolution of DNA single-strand breaks involves specialized factors that process and repair specific types of breaks. Direct DNA breaks (which may arise from radiation, oxidation or abortive Top1-DNA intermediates) undergo detection by PARP, which signals the breaksite through poly-ADP ribosylation of XRCC1 and DNA end-processing factors. The formation of ADP-ribose polymers on these proteins serves to augment affinity and facilitate protein complex recruitment to the DNA breaksite. Damaged 5′- and 3′-end DNA termini undergo end-modification by DNA processing factors to generate 5′- end phosphate and 3′-end hydroxyl termini, which allows DNA polymerase to replace the missing nucleotide(s) and DNA ligase to restore integrity of the DNA strand backbone. DNA short-patch repair involves integration of a single nucleotide via DNA polymerase β into the damaged strand and DNA ligation by XRCC1-bound LIG3. Indirect DNA breaks arise from enzymatic excision of apurinic-apyrimidinic (AP) bases (which can occur from base alkylation) via APE1 or DNA glycosylases. Although this substrate can undergo XRCC1-mediated end-processing, followed by DNA short-patch repair, certain classes of damage undergo long-patch DNA repair, whereby DNA polymerase δ and ε generate an extended nucleotide polymer (2–12 nucleotides) to replace DNA bases at and proximal to the DNA break site. Flap-endonuclease 1 (FEN1) cleaves the extraneous DNA flap, and the intact DNA backbone is ligated via PCNA-bound LIG1. The shaded box represents the aspect of SSBR involving LIG3, which is now less clear based on new dataCitation44,Citation65 discussed in this paper.
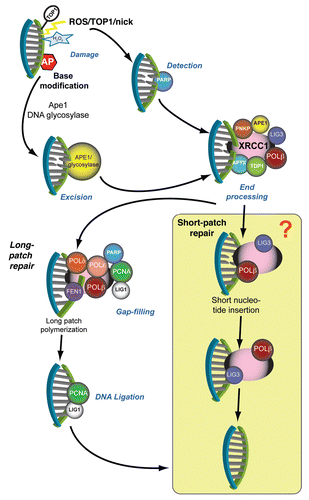
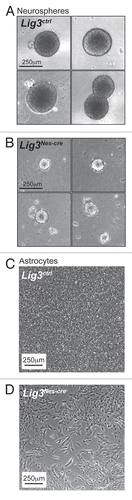
Figure 2 Primary Lig3Nes-cre neural cell lines have growth defects. Photomicrographs of primary neurospheres derived from (A) Lig3ctrl and (B) Lig3Nes-cre neural stem cells isolated from E14.5 mouse embryos. Lig3Nes-cre neurospheres are significantly smaller and display reduced cellularity compared to WT (Lig3ctrl) counterparts. (C) Primary astrocytes isolated from WT P3 mice form a tight confluent monolayer five days post-isolation. (D) In contrast, Lig3Nes-cre astrocytes cultures are growth delayed.