Figures & data
Figure 1 Spatio-temporal formation and resolution of DNA repair foci induced by ionizing radiation. (A) Lmna+/+ and Lmna−/− MEFs were irradiated with 0.5 Gy or left untreated. Cells were fixed at different times post-IR (from 30 min to 24 h) and subjected to immunofluorescence (IF) with an antibody recognizing 53BP1. Pictures of fields selected randomly were taken under the same conditions of exposure. Representative images of four cells per condition are shown in each part. (B) Lmna+/+ and Lmna−/− MEFs were irradiated and at different times post-IR cells were fixed and subjected to IF with an antibody recognizing γH2AX. Cells presenting more than 5 γH2AX foci were scored as positive for responding to IR. A total of 200–300 cells were scored per time point and per experiment. The average ± standard deviation of three independent experiments is shown.
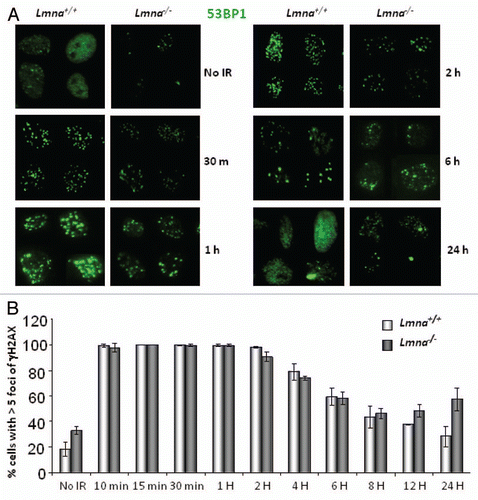
Figure 2 Defective repair of IR-induced DNA DSBs upon loss of A-type lamins. (A) Asynchronously growing cells were irradiated with 8 Gy. At different times post-irradiation (0, 30, 60, 90, 120 and 150 min) cells were collected and neutral comet assays were performed. Kinetics of repair of IR-induced DSBs was assessed by Olive Comet Moment, a measure of unrepaired DNA. A total of 25 to 30 cells were analyzed per sample and per time-point. (B) Protein gel blot to monitor the levels of 53BP1 protein in Lmna+/+ and Lmna−/− MEFs retrovirally transduced with EV control or a 53BP1-expressing vector. Short and long exposures of 53BP1 are shown. Actin levels are used as loading control.
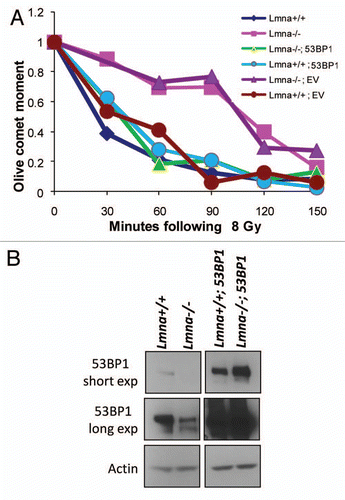
Figure 3 Defects in NHEJ of dysfunctional telomeres are rescued by reconstitution of 53BP1. (A) Levels of 53BP1 and A-type lamins in U2OS cells after ectopic expression of 53BP1 (EV, empty vector control), followed by lentiviral transduction with shLmna or shCtrl. β-tubulin was used as loading control. Note how ectopic expression of 53BP1 prevents the decrease in protein levels upon depletion of A-type lamins. (B) Key of the different categories of metaphases based on the extent of chromosome end-to-end fusions induced by expression of TRF2ΔBΔM. Representative images of the different categories are shown. (C) Histograms showing the percentage of metaphases belonging to each category from the different cell lines. Top part: cells transduced with EV, shCtrl and TRF2ΔBΔM. Middle part: cells transduced with EV, shLmna and TRF2ΔBΔM. Bottom part: cells transduced with 53BP1, shLmna and TRF2ΔBΔM. 79–88 metaphases were analyzed per condition.
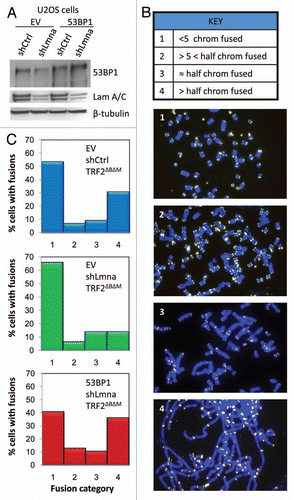
Figure 4 A-type lamins promote HR by maintaining RAD51 levels and recruitment to DSBs. (A) Protein gel blots showing decreased 53BP1 protein upon depletion of A-type lamins in MCF-7 cells carrying an HR reporter construct (DR-GFP). (B) Percent of GFP-positive MCF7-DR-GFP cells resulting from HR of I-SceI-induced DSBs. Depletion of A-type lamins leads to a 40% reduction in HR. (C) Percentage of MCF7-DR-GFP cells positive for RAD51 foci (more than 10 foci throughout the nucleus) 6 h after treatment with 8 Gy. A total of 200 cells per condition were analyzed per experiment. The average ± standard deviation of three independent experiments is shown. (D) Representative images of RAD51 foci. Blue images show DAPI stained nuclei, green shows RAD51 IF. (E) Protein gel blots showing the decrease in global levels of RAD51 upon depletion of A-type lamins in MCF-7 DR-GFP cells. β-tubulin was used as loading control. (F) Protein gel blots showing lower RAD51 upon depletion of A-type lamins in MEFs. (G) Percentage of MEFs positive for RAD51 foci 6 h after treatment with 8 Gy. A total of 290-10 cells per condition were analyzed.
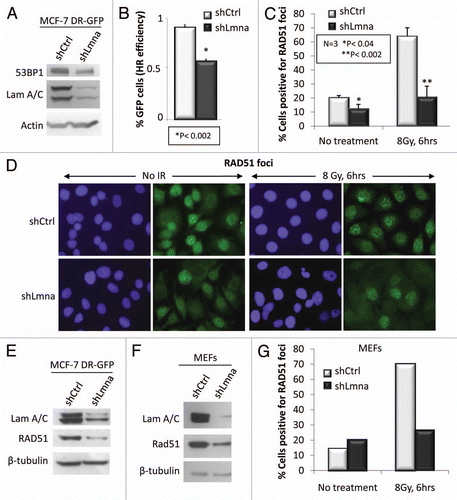
Figure 5 Loss of A-type lamins significantly affects transcriptional regulation of RAD51 and BRCA1. (A) Protein gel blots showing lower levels of BRCA1, but not RPA2 in cells depleted of A-type lamins. β-tubulin was used as loading control. (B) Percent of cells positive for formation of RPA2 foci 6 h after treatment with 8 Gy IR. (C) qRT-PCR to determine transcript levels of RAD51, BRCA1 and RPA2. Shown are the averages and standard deviations from three independent experiments.
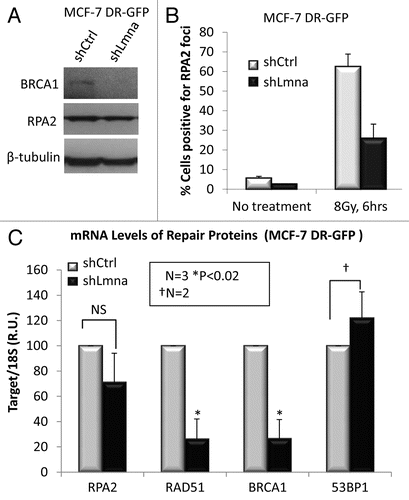
Figure 6 Decreased RAD51 in lamins A/C-deficient cells requires p130, a member of the pocket family of proteins. (A) Protein gel blot showing depletion of A-type lamins from MEFs that are null for two (DKO: Rb−/−; p107−/−) or all three (TKO: Rb−/−; p107−/−; p130−/−) of the pocket family proteins. Note how loss of lamins leads to a dramatic decrease of RAD51 only in the cells that are p130 proficient (DKO). (B) Co-immunoprecipitation of p130 with E2F4. Lystates from lamins A/C-proficient or -deficient cells were subjected to immunoprecipitation with an E2F4 antibody. Protein gel blots (left) shows successful immunoprecipitation of E2F4 and co-precipitation of p130. Inputs are shown on the right part.
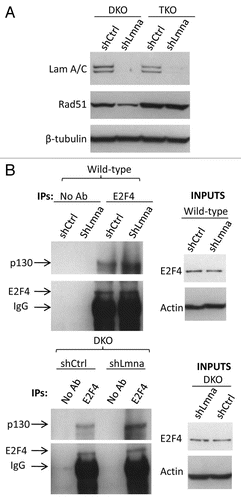
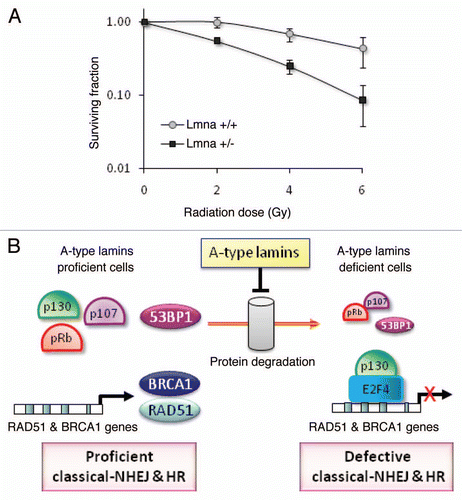
Figure 7 Radiosensitivity and model of the dual role of A-type lamins in DNA DSBs repair. (A) Clonogenic survival in Lmna+/+ and Lmna−/− MEFs in response to increasing doses of radiation (0 to 6 Gy). Shown are the surviving fractions and standard deviation of the mean from three independent experiments. (B) A-type lamins play a role in the stabilization of the pocket family proteins pRb and p107, as well as 53BP1, in part by preventing their degradation by the proteasome. By stabilizing 53BP1, A-type lamins promote classical-NHEJ. In addition, A-type lamins regulate transcriptionally two key factors in HR, RAD51 and BRCA1. Loss of A-type lamins leads to increased formation of p130/E2F4 complexes, which in turn can bind the RAD51 and BRCA1 gene promoters and inhibit their transcription. Loss of A-type lamins leads to defects in the two major mechanisms of DNA DSBs repair (NHEJ and HR), increased genomic instability and radiation sensitivity.