Figures & data
Figure 1 Overall strategy for quantitating cancer metabolism in MCF7-fibroblast co-cultures. MCF7 cells are co-cultured with stromal fibroblasts for 5 d. Then, the co-cultures are briefly incubated with different fluorescent probes to measure mitochondrial activity (Mito-Tracker), glucose uptake (NBD-2-deoxy-glucose) or ROS production (DCF-DA). Afterwards, co-cultures are mildy-trypsinized and subjected to FACS analysis to physically separate MCF7 cancer cells from stromal fibroblasts which is analogous to laser-capture micro-dissection (LCM). This strategy allows for compartment-specific metabolic analysis. For all of our experiments, we used MCF7 cells recombinantly tagged with either GFP (green fluorescent protein) or RFP (red fluorescent protein) and unlabelled stromal fibroblasts.
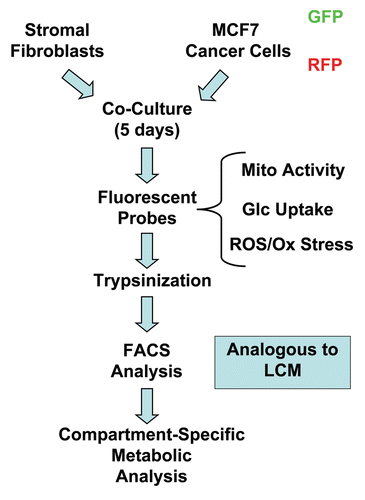
Figure 2 Changes in mitochondrial activity in MCF7 cells and fibroblasts in co-culture. FACS tracings for mitochondrial activity (MitoTracker red fluorescence) in fibroblasts or cancer cells, either cultured alone or in combination, are shown. Interestingly, during co-culture, MCF7 cells show a shift to the right, as indicated by the blue arrow. Conversely, during co-culture, stromal fibroblasts show just the opposite effect, with a shift to the left.
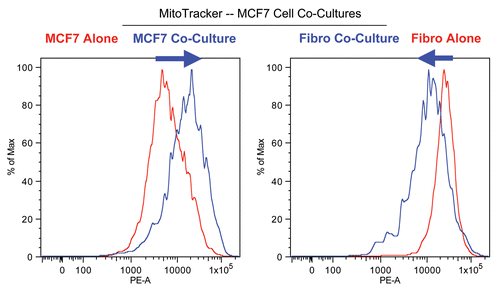
Figure 3 Increased mitochondrial activity in MCF7 cells in co-culture: quantitative analysis. After 5 d of co-culture, MCF7 cells show a >2-fold increase in mitochondrial activity (A). Similar results were obtained using a range (10–35 nM) of Mitotracker concentrations (B).
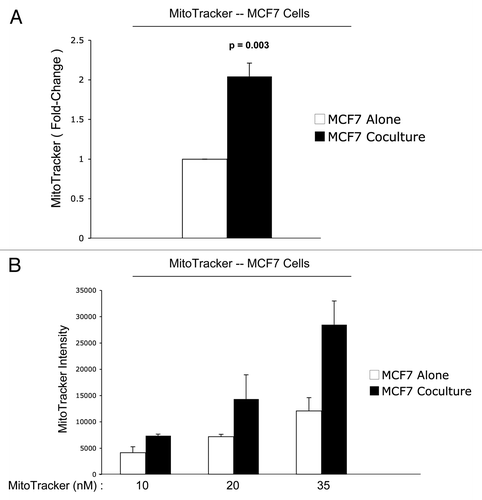
Figure 4 Reduced mitochondrial activity in fibroblasts in co-culture: quantitative analysis. After 5 d of co-culture, stromal fibroblasts show a 1.7-fold reduction in mitochondrial activity (A). Similar results were obtained using a range (10–35 nM) of MitoTracker concentrations (B).
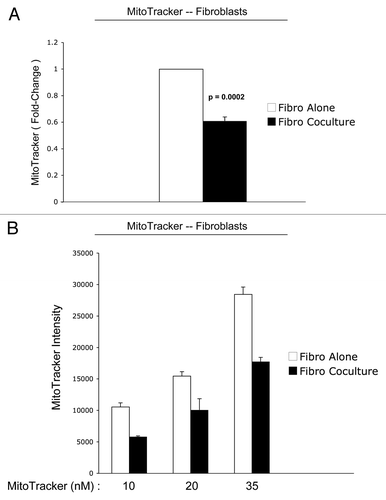
Figure 5 Mitochondrial activity in MDA-MB-231 cells and fibroblasts in co-culture. FACS tracings for mitochondrial activity (MitoTracker red fluorescence) in fibroblasts or MDA-MB-231 cancer cells, either cultured alone or in combination, are shown (A). The direction of change in co-culture is indicated by blue arrows. Interestingly, after 5 d of co-culture, MDA-MB-231 cells show a shift to the right, which corresponds to a 1.5-fold increase in mitochondrial activity (A and B). Conversely, after 5 d of co-culture, stromal fibroblasts show just the opposite effect, with a shift to the left, resulting in a >5-fold reduction in mitochondrial activity (A and C).
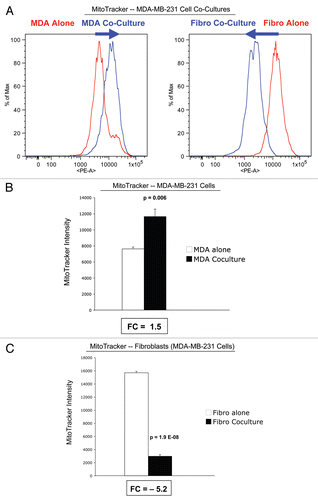
Figure 6 Glucose uptake in MCF7 cells and fibroblasts in co-culture. FACS tracings for glucose uptake (NBD-2-deoxy-glucose) in fibroblasts or MCF7 cancer cells, either cultured alone or in combination, are shown (A). The direction of change in co-culture is indicated by blue arrows. Note that after 5 d of co-culture, stromal fibroblasts show a shift to the right, which corresponds to a 1.8-fold increase in glucose uptake (A and B). Remarkably, after 5 d of co-culture, MCF7 cancer cells show just the opposite phenotype, with a shift to the left, resulting in a 1.6-fold reduction in glucose uptake (A and B).
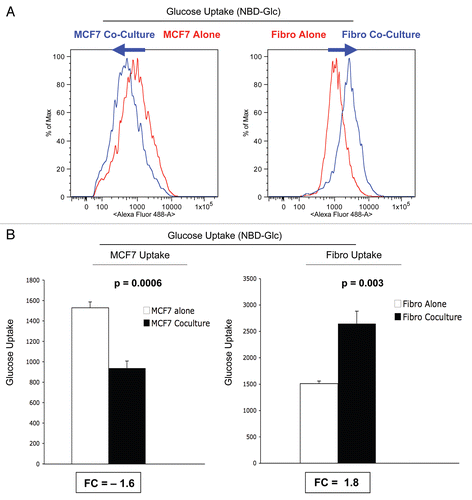
Figure 7 ROS production in MCF7 cells and fibroblasts at 5-days of co-culture. MCF7 cancer cells and stromal fibroblasts were co-cultured for 5 d, and then subjected to FACS analysis, with the fluorescent probe DCF-DA, which measures ROS production. FACS tracings for ROS production are shown in (A). At day 5 of co-culture, stromal fibroblasts show a shift to the right, which corresponds to a >two-fold increase in ROS production (B). In contrast, cancer cells show only a 1.2-fold increase in ROS production, under the same conditions (B). Thus, after 5 d of co-culture, stromal fibroblasts show increases in ROS production and glucose uptake, and a corresponding decrease in mitochondrial activity.
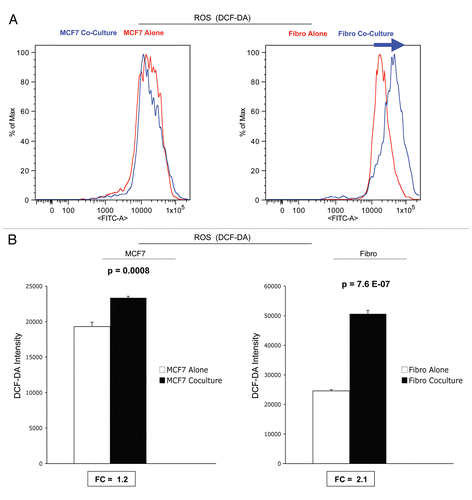
Figure 8 ROS production in MCF7 cells and fibroblasts at 2-days of co-culture: effects of catalase. At 2 d of co-culture, MCF7 cancer cells show a near three-fold induction of ROS production (A), while stromal fibroblasts have only minor increases in ROS production (B). Note that addition of extracellular catalase (an enzyme that detoxifies hydrogen peroxide to water) was sufficient to normalize ROS associated with co-cultured cancer cells (A). Under these conditions, an irrelevant protein (namely BSA) had no effect (A). Thus, it appear that ROS production starts in cancer cells, via the generation and secretion of hydrogen peroxide, and then spreads to adjacent stromal fibroblasts.
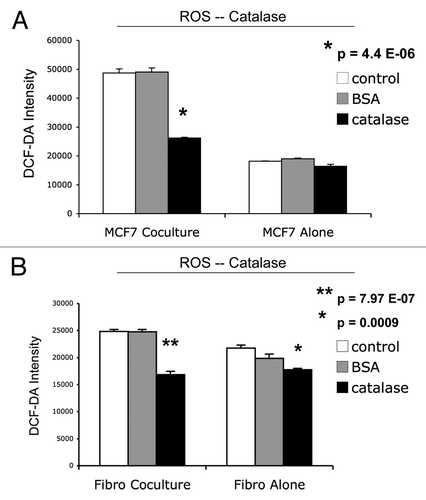
Figure 9 Catalase induces apoptosis in MCF7 cells in co-culture. Next, we added catalase to MCF7-fibroblas co-culture to detoxify hydrogen peroxide and prevent oxidative stress. Note that the simple addition of extracellular catalase to the culture media induced a five-fold increase in apoptotic cell death in co-cultured cancer cells (A); however, an irrelevant protein (namely BSA) had no effect (B). Catalase may induce apoptosis in co-cultured cancer cells, by detoxifying extracellular hydrogen peroxide, thereby cutting off the fuel supply for cancer cells. Cell death was defined as the Annexin V(+)/PI(−) population of cells (“early apoptosis”).
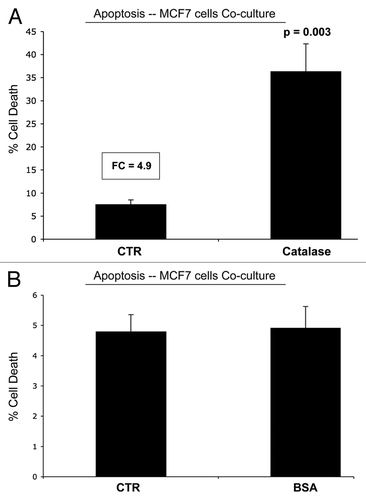
Figure 10 GLUT1 expression in MCF7 cells is downregulated by co-culture with stromal fibroblasts. MCF7 cell-fibroblast co-cultures were immuno-stained with a specific antibody probe directed against GLUT1. MCF7 cells cultured alone were processed in parallel, under identical culture conditions. Note that GLUT1 expression is dramatically downregulated when MCF7 cells are co-cultured with fibroblasts, consistent with our functional results obtained by FACS analysis. MCF7 cells were visualized by immuno-staining with keratin-8/18 antibodies. Nuclei are counter-stained with DAPI and appear blue.
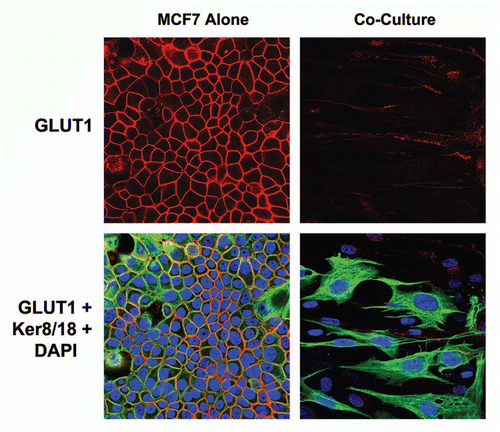
Figure 11 GLUT1 expression in MCF7 cells is downregulated by high energy metabolites. MCF7 cells alone were treated with L-Lactate (10 mM) and 3-Hydroxy-Butyrate (10 mM) for 2 d or left untreated. Note that treatment with either L-Lactate and 3-Hydroxy-Butyrate is sufficient to phenocopy the effects of fibroblasts on GLUT1 expression in co-cultured MCF7 cells, resulting in a loss of GLUT1 expression.
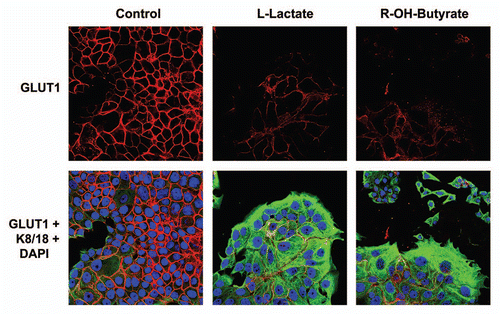
Figure 12 A loss of Cav-1 drives mitochondrial dysfunction and increased glucose uptake in stromal fibroblasts. (A) Western blot analysis shows the targeted stable knock-down of Cav-1 in human stromal fibroblasts, using an siRNA-based approach. Immuno-blotting with β-actin is shown as a control for equal loading. (B) Cav-1 knock-down fibroblasts show a ∼1.8 fold reduction in mitochondrial activity, as measured with the MitoTracker probe, relative to control vector fibroblasts. (C) Cav-1 knock-down fibroblasts show a ∼1.4-fold increase in glucose uptake, using NBD-2-deoxy-glucose, relative to control vector fibroblasts.
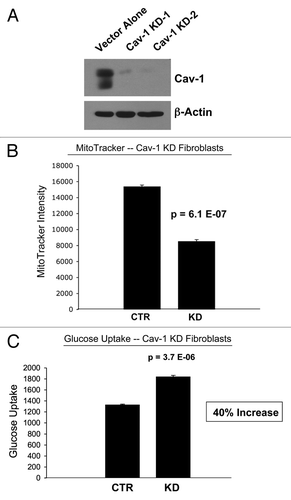
Figure 13 Hydrogen peroxide and ROS production in the tumor micro-environment fuels the anabolic growth of cancer cells. Recently, we proposed a new mechanism for understanding the Warburg effect in tumor metabolism. In this new paradigm, cancer cells induce oxidative stress in neighboring cancer-associated fibroblasts. Then, oxidative stress, in cancer-associated fibroblasts, triggers the activation of two main transcription factors, NFκB and HIF-1α, leading to the onset of inflammation, autophagy, mitophagy and aerobic glycolysis in the tumor micro-environment. this type of stromal metabolism then produces high-energy nutrients (lactate, ketones and glutamine), as well as recycled chemical building blocks (nucleotides, amino acids, fatty acids), to literally “feed” cancer cells. these nutrients (such as lactate) stimulate mitochondrial biogenesis in adjacent cancer cells. In addition, the oxidative stress and ROS, produced in cancer-associated fibroblasts, has a “bystander effect” on adjacent cancer cells, leading to DNA damage, genomic instability and aneuploidy, driving tumor-stroma co-evolution. We have previously termed this new paradigm the “reverse Warburg effect,” as we see that aerobic glycolysis takes place in cancer-associated fibroblasts, rather than in tumor cells, as previously suspected. Here, we showed that tumor cells produce and secrete hydrogen peroxide, thereby “fertilizing” the tumor microenvironment and driving the “reverse Warburg effect.” As a consequence, oxidative stress initiated in tumor cells is transferred to cancer-associated fibroblasts, via hydrogen peroxide. Modified and reproduced with permission from reference Citation6.
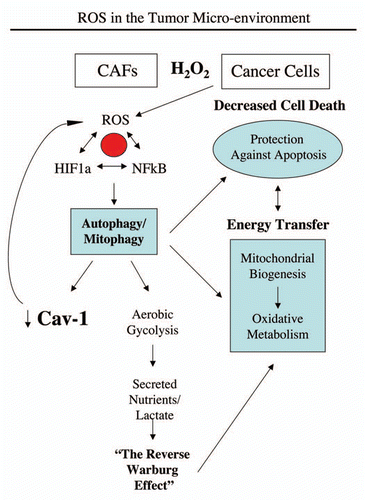
Table 1 Relative mitochondrial activity, glucose uptake and ROS production in single cell cultures vs. co-cultures