Figures & data
Figure 1 Cisplatin induced miR-885-3p expression through p-ΔNp63α. (A) qPCR analysis of the miR-885-3p levels in SCC-wt-ΔNp63α or SCC-ΔNp63α-S385G cells. Cells were incubated with control medium or 10 µg/ml cisplatin for 16 h. miRNA levels were monitored by qPCR. Data were normalized against the glyceraldehyde-3-phospho dehydrogenase levels and plotted as relative units (RU) with measurements obtained from wt-ΔNp63α cells treated with control medium set as 1. Experiments were performed in triplicate with +SD as indicated (p < 0.01). (B–I) Representation of the consensus miR-885-3p sequence (B) and “recognition” sequences in the 5′-UTR of Mdm4 (C), 3′-UTR of Akt1 (D), 3′-UTR of Bcl2 (E), 3′-UTR of Atg16L2 (F), 3′-UTR of Ulk2 (G), 3′-UTR of Casp2 (H) and 3′-UTR of Casp3 (I). The miR-885-3p consensus sequence and 5′-UTR/3′-UTR “recognition” sequences were obtained from the www.microrna.org web database. The capital letters indicate the nucleotides in the target mRNA sequences that are complementary to the nucleotides in the miR-885-3p consensus sequence.
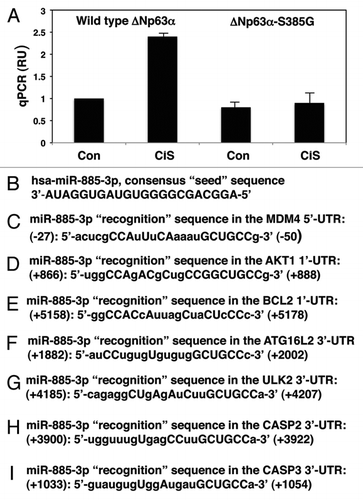
Figure 2 Cisplatin exposure modulated the miR-885-3p-mediated effect on specific target mRNAs. SCC-wt-ΔNp63α cells were transfected for 24 h with the luciferase reporter plasmids with 5′-UTR of Mdm4 (A), 3′-UTR of Akt1 (B) and 3′-UTR of Bcl2 (C). Each UTR sequence was found to contain the specific “recognition” sequences, which potentially could form double-stranded complexes with the miR-885-3p consensus sequence. Cells were also transfected for 24 h with the scrambled RNA and an inhibitor or mimic for miR-885-3p. Resulting cells were further incubated with control medium or 10 µg/ml cisplatin for an additional 16 h, as indicated. The luciferase activity was measured at 480 nm using a luminometer. Each experiment was performed independently at least three times and in triplicate with +SD as indicated (p < 0.01).
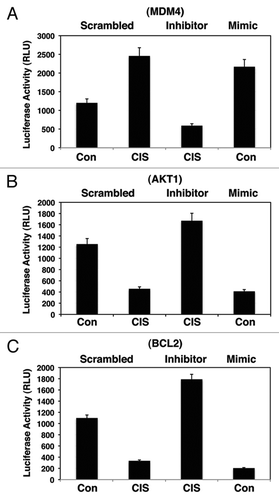
Figure 3 Cisplatin exposure modulated the miR-885-3p-mediated effect on specific target mRNAs. SCC-wt-ΔNp63α cells were transfected for 24 h with the luciferase reporter plasmids with 3′-UTR of Atg16L2 (A), 3′-UTR of Ulk2 (B), 3′-UTR of Casp2 (C) and 3′-UTR of Casp3 (D). Each UTR sequence was found to contain the specific “recognition” sequences, which potentially could form double-stranded complexes with the miR-885-3p consensus sequence. Cells were also transfected for 24 h with the scrambled RNA and an inhibitor or mimic for miR-885-3p. Resulting cells were further incubated with control medium or 10 µg/ml cisplatin for an additional 16 h, as indicated. The luciferase activity was measured at 480 nm using a luminometer. Each experiment was performed independently at least three times and in triplicate with +SD as indicated (p < 0.01).
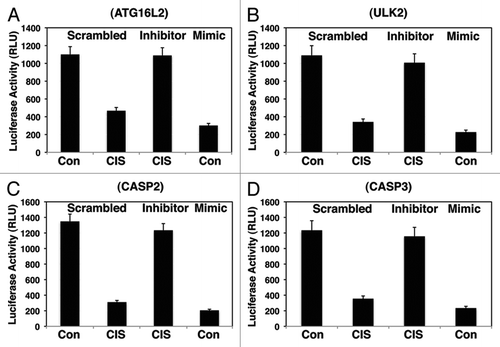
Figure 4 Cisplatin induced the p-ΔNp63α mediated modulation of target protein levels through miR-885-3p. SCC-wt-ΔNp63α cells (left parts) and SCC-ΔNp63α-S385G cells (right parts) were transfected for 24 h with the scrambled RNA and an inhibitor or mimic for miR-885-3p. Resulting cells were further incubated with control medium or 10 µg/ml cisplatin for an additional 16 h, as indicated. Protein expression was tested in total lysates using the indicated antibodies, while loading was monitored by anti-β-actin antibody.
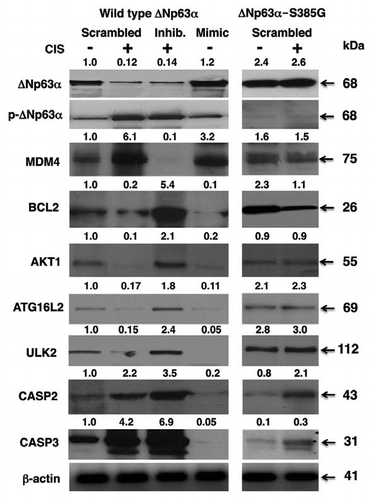
Figure 5 Cisplatin modulated cell viability via p-ΔNp63α/miR-885-3p axis. SCC-wt-ΔNp63α cells (A) and SCC-ΔNp63α-S385G cells (B) were transfected with the miR-885-3p inhibitor (curve 3) or miR-885-3p mimic (curve 4) for 24 h. Cells were then exposed to control medium (Con, curves 1 and 4) or 10 µg/ml cisplatin (CIS, curves 2 and 3) for an additional 0–120 h. Cell survival was monitored at 24, 48, 72, 96 and 120 h by measuring the mitochondrial activity by MTT reagent. Experiments were performed in triplicate with +SD as indicated (p < 0.05). In parallel experiments, SCC-wt-ΔNp63α cells were transfected with Mdm4 siRNA, pCMV6-Akt1, pCMV6-Bcl2 and pCMV6-Ulk2 (C), while SCC-ΔNp63α-S385G cells were transfected with pCMV6-Mdm4, siRNA against Akt1, Bcl2 or Ulk2 (D) for 24 h. Cells then were exposed to control medium or 10 µg/ml cisplatin for an additional 48 h. Cell viability was monitored at 72 h by measuring the mitochondrial activity by MTT reagent. Experiments were performed in triplicate with + SD, as indicated (p < 0.05).
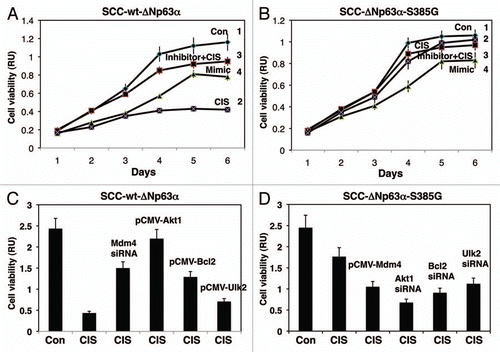
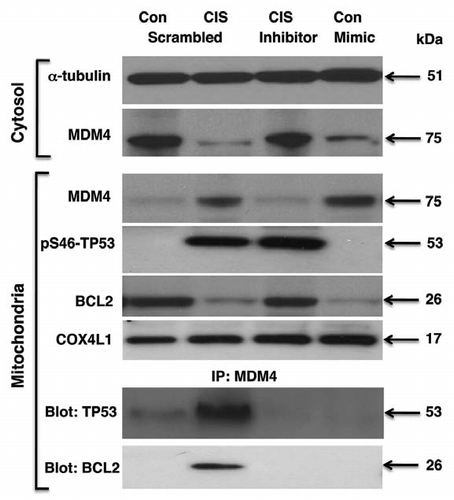
Figure 6 Cisplatin induced miR-885-3p-modulated MDM4/p-S46-TP53/BCL2 protein interactions. Wt-ΔNp63α cells were transfected with scrambled RNA and an inhibitor or mimic for miR-885-3p for 24 h. Resulting cells were exposed to control medium and 10 µg/ml of cisplatin for an additional 16 h. Protein levels for MDM4, BCL2 and p-S46-TP53 in cytosol (cyto) and mitochondria were examined by immunoblotting with the indicated antibodies. Protein loading levels were tested with anti-α-tubulin and anti-cytochrome c oxidase (COX4L1) antibodies for cytosol (cyto) and mitochondria, respectively. Mitochondrial lysates were immunoprecipitated (IP) with antibody to MDM4 and then blotted with antibodies to TP53 and BCL2.