Figures & data
Figure 1 TFAM-deficient fibroblasts show a loss of Caveolin-1 protein expression. We generated TFAM-deficient immortalized fibroblast cell lines (hTERT-BJ1), using an sh-RNA approach. Note the successful knock-down of TFAM in stromal fibroblasts (sh-TFAM), as compared to control fibroblasts (sh-Ctrl), as seen by immuno-blot analysis. We also examined the status of the caveolin-1 (Cav-1) protein, because a loss of Cav-1 expression in the tumor stroma is a biomarker for tumor progression. Immuno-blot analysis shows that downregulation of TFAM also results in the loss of Cav-1 protein expression. Blotting for β-actin was performed in parallel as a control for equal protein loading.
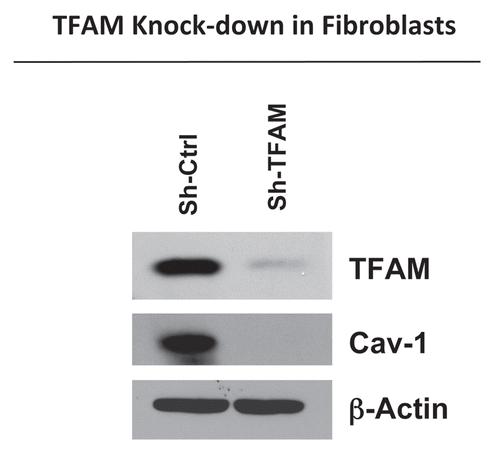
Figure 2 TFAM-deficient fibroblasts show defects in oxidative phosphorylation. To assess the status of the mitochondrial respiratory chain in fibroblasts, a battery of antibodies directed against mitochondrial complex components (I–V) were examined. Under normoxic conditions, no changes were observed between sh-TFAM and sh-Ctrl fibroblasts. However, under hypoxic conditions, note the decrease in Complexes I–IV. Immunoblotting with β-actin is shown as a control for equal loading.
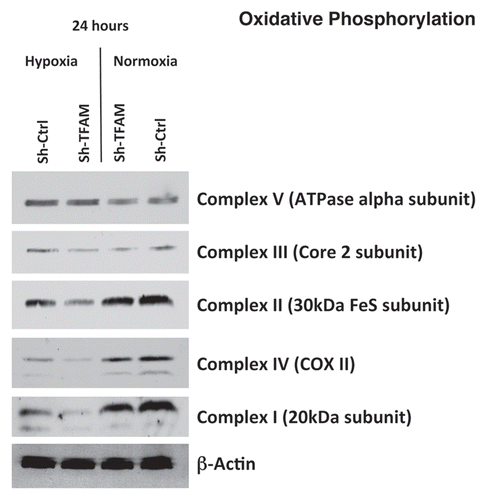
Figure 3 TFAM-deficient fibroblasts show a decrease in functional mitochondrial activity. We examined functional mitochondrial activity under normoxic conditions and found that sh-TFAM fibroblasts show a small, but significant, reduction (∼12%; p = 0.005) in activity.
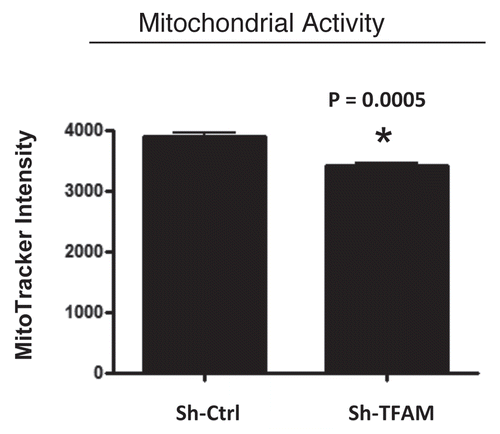
Figure 4 TFAM-deficient fibroblasts produce more hydrogen peroxide. Hydrogen peroxide production is significantly increased (∼2-fold; p = 0.001) in sh-TFAM fibroblasts, as compared to sh-Ctrl fibroblasts. The ability of TFAM-deficient fibroblasts to produce excess hydrogen peroxide suggests that a loss of TFAM expression could mimic the phenotypic behavior of tumor-associated myo-fibrobasts undergoing oxidative stress.
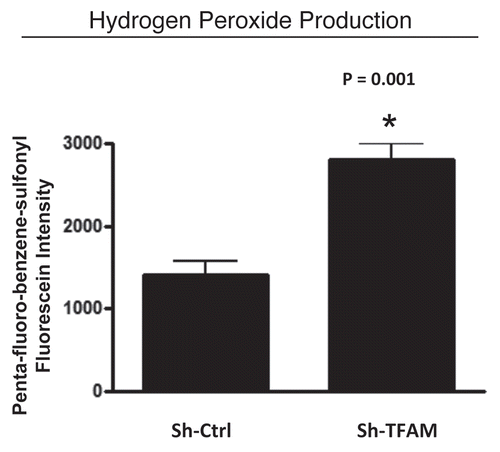
Figure 5 TFAM-deficient fibroblasts secrete elevated levels of L-lactate, consistent with increased aerobic glycolysis. L-lactate is a critical fuel which provides continued energetic support for tumor epithelial cells. Note that TFAM-deficient fibroblast secrete increased levels of L-lactate (∼2-fold; p = 0.006), relative to control fibroblasts processed in parallel.
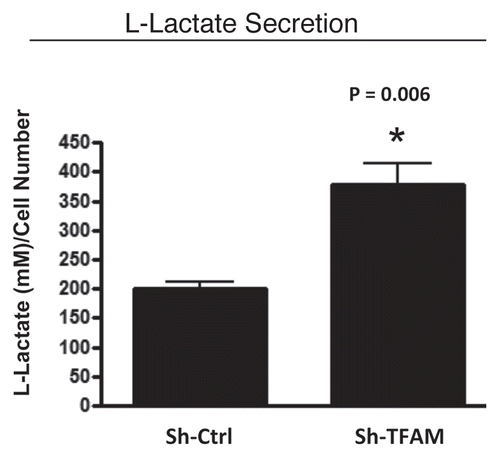
Figure 6 TFAM-deficient fibroblasts promote tumor growth. We used an in vivo murine xenograft model to evaluate the tumor-promoting properties of TFAM-deficient fibroblasts. Fibroblasts were co-injected with MDA-MB-231 breast cancer cells into the flanks of immuno-deficient nude mice. After 4 weeks, the tumors were harvested and subjected to detailed analysis. sh-TFAM fibroblasts were able to promote tumorigenesis, with an up to 2-fold increase in tumor growth. This represents an ∼1.6-fold increase (p = 0.03) in tumor weight and an ∼2.1-fold increase (p = 0.006) in tumor volume.
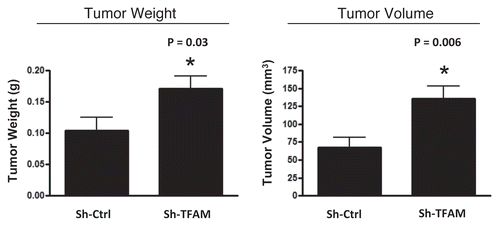
Figure 7 TFAM-deficient fibroblasts do not increase tumor angiogenesis. We used an in vivo murine xenograft model to evaluate the tumor-promoting properties of TFAM-deficient fibroblasts. Fibroblasts were co-injected with MDA-MB-231 breast cancer cells into the flanks of immuno-deficient nude mice. After 4 weeks, the tumors were harvested and subjected to detailed analysis. There were no observed differences in tumor vessel density density (number of vessels per field), as measured using CD31 immuno-staining. As such, the tumor growth promoting activity of TFAM-deficient fibroblasts is independent of tumor angiogenesis.
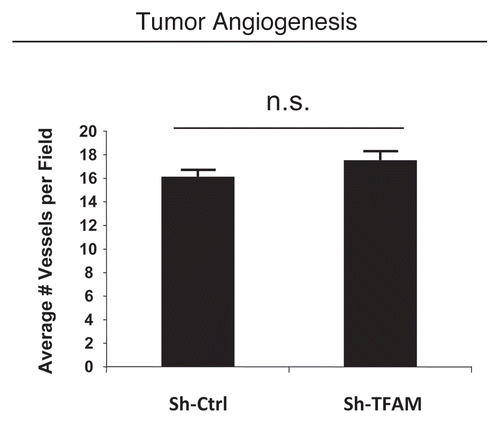
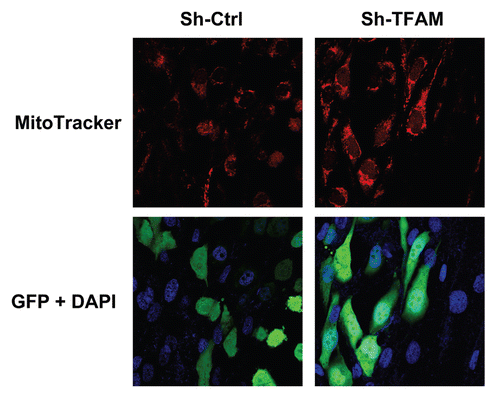
Figure 8 TFAM-deficient fibroblasts increase mitochondrial activity in adjacent cancer epithelial cells. We co-cultured sh-TFAM and sh-Ctrl fibroblasts with GFP-tagged MDA-MB-231 breast cancer cells. Then, we visualized mitochondrial activity in these co-cultures using MitoTracker. Note that TFAM-fibroblasts specifically increase the mitochondrial activity of adjacent MDA-MB-231 cells. MitoTracker (red); epithelial cancer cells/GFP (green); nuclei/DAP I (blue). See online version for color version of the figure.