Figures & data
Figure 1 BMAL1 deficiency leads to accumulation of senescent cells in vivo. Representative histological images of the lungs (left column), liver (middle column) and spleen (right column) isolated from 6 mo old wildtype (upper row) or Bmal1-/- (lower row) mice were stained for senescent associated β-galactosidase.
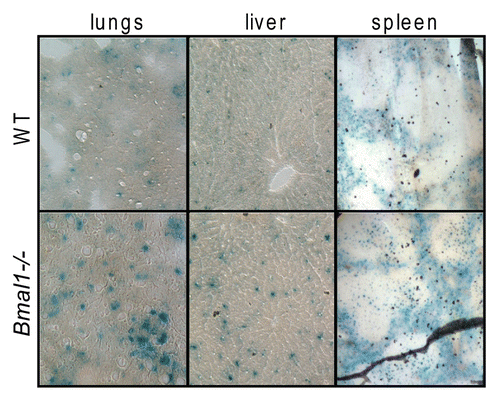
Figure 2 BMAL1 deficiency does not affect fibroblast replicative senescence in vitro. Growth curves of primary lung fibroblasts isolated from wild type (closed circles) and Bmal1-/- (open circles). On indicated days cells were counted and plated with equal density.
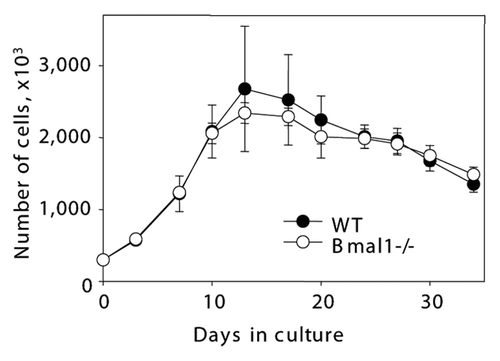
Figure 3 BMAL1-deficient cells have increased sensitivity to hydrogen peroxide. Relative survival of wild-type (black circles) and Bmal1-/- (open circles) fibroblasts treated with indicated concentrations of hydrogen peroxide for 24 h (A), 48 h (B) and 72 h (C). Survival of untreated cells was set as 100%. *p < 0.01.
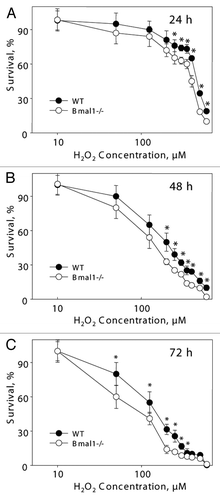
Figure 4 Increased sensitivity of BMAL1-deficient cells to hydrogen peroxide is due to increased growth inhibition. (A) Relative cell death of wildtype (black circles) and Bmal1-/- (open circles) fibroblasts treated with indicated concentrations of hydrogen peroxide for 4 h were calculated based on fluorescent signal using kit. Signal of untreated cells was set as 0 and signal of cells lysed with SDS was set as 1.0. (B) Numbers of colonies formed by wild-type (black bars) and Bmal1-/- (gray bars) fibroblasts. Equal amount of cells were plated, cells grew in regular growth media for 96 h (U/T), or in the presence of 250 mM of hydrogen peroxide for 96 h (250 mM H2O2), or treated with 250 mM of hydrogen peroxide for 24 h and then in regular growth media for additional 72 h (250 mM H2O2/media). (C) Representative images of colony formation experiment. *p < 0.05.
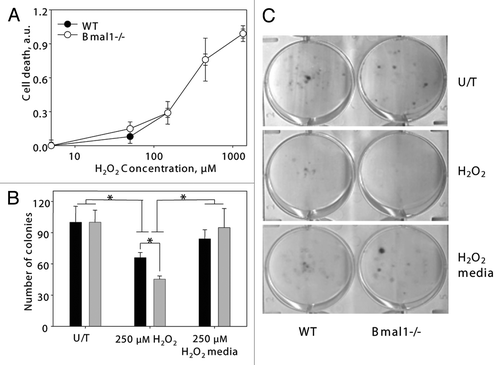
Figure 5 BMAL1 deficiency cells have differential sensitivity to anticancer drugs. Relative survival of wild-type (black circles) and Bmal1-/- (open circles) fibroblasts treated with indicated concentrations of paclitaxel (A), 5-fluorouracil (B), daunorubicin (C) and etoposide (D). Survival of untreated cells was set as 100%. *p < 0.01.
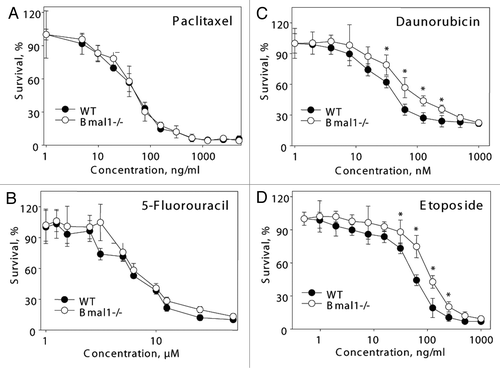
Figure 6 Increased sensitivity of BMAL1-deficient cells to hydrogen peroxide is p53-independent. Relative survival of GSE 56 (dominant negative fragment of p53) wild-type (black circles) and Bmal1-/- (open circles) fibroblasts treated with indicated concentrations of hydrogen peroxide for 72 h. Survival of untreated cells was set as 100%. *p < 0.01.
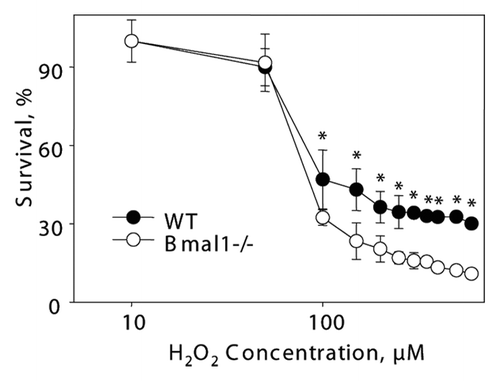
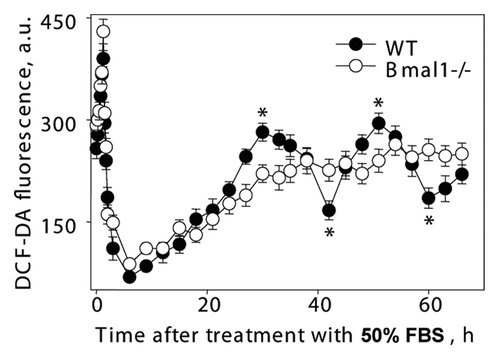
Figure 7 Circadian rhythms of intracellular ROS level can be induced in cell culture. Confluent wild-type (black circles) and Bmal1-/- (open circles) fibroblasts were maintained in DMEM with 5% FBS for 7 d, at time 0 h media were replaced with media with 50% FBS (serum shock); cells were incubated for 2 h and then 50% FBS media was replaced with serum free media; cells were collected every 10 min during serum shock and every 3 h after serum replacement and ROS level was measured as described in Materials and Methods. *Statistically significant (p < 0.001) difference between peak and throw for wild-type cells.