Figures & data
Figure 1 Visualization of CreER-induced gene recombination in adult cochlea with a GFP reporter. GFP expression is shown in confocal images from whole-mount preparations of apical turn organ of Corti from 6 week-old tamoxifen-injected (group G6 animal 1, A–C; animal 2, D–F) or vehicle only (oil, G and H) animals. Tissues are labeled with Sox2 antibody (red) that specifically labels support cells and GFP (green), plus a nuclear counterstain, DAPI (blue).Citation28 Panels show a brightest-point projection of a confocal Z-series spanning the sensory epithelium. GFP label alone, in grayscale, is shown in the middle panels (B, E and H) to illustrate the morphology of the GFP-positive cells. Only Sox2 and DAPI are shown in the right panels, demonstrating 3 rows of Sox2-negative outer hair cell nuclei (OHC1–3) and 1 row of inner hair cell nuclei (IHC). Many GFP-positive support cells and hair cells are present in the CreER+ animals treated with tamoxifen (A–F). (G and H) A rare GFP-positive IHC (arrow) present in a vehicle (oil) treated adult control mouse. Scale bar = 10 µm (A–C) or 20 µm (D–H).
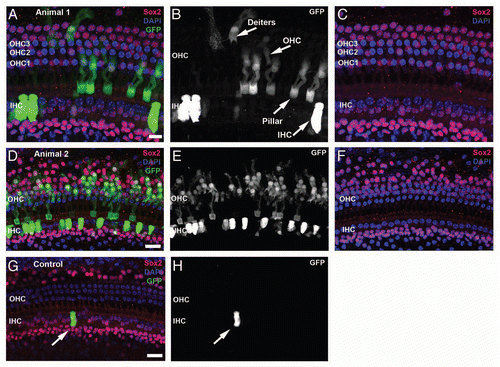
Figure 2 Proliferation of neonatal organ of Corti cells after induced deletion of p27Kip1. Confocal images from cochlea of neonatal mice given BrdU (group L1) are shown at the level of HC (left column) or Deiters/pillar nuclei (right column). Control animals, including those given oil (without tamoxifen, A and B) and tamoxifen-treated p27 wild-type mice (C and D), show Sox2 staining (red) in the greater epithelial ridge (GER) and in SCs cells. One row of inner (IHC) and 3 rows of outer (OHC) hair cells are Sox2- and seen with DAPI nuclear staining (blue). BrdU staining (green) is not seen in the organ of Corti or GER but is present in the Sox2- Claudius cells (arrow, D) and in the underlying stroma (B). In the brightest-point projection shown in (B), the BrdU labeling that appears in the GER is actually in underlying BrdU-labeled stromal cells; the GER cells are unlabeled. (E and F) Experimental animals (p27L+/L+;CreER+) given tamoxifen and BrdU show numerous Sox2+/BrdU+ cells in both the organ of Corti and GER. (G and H) Constitutive p27 knockout animal (positive control) treated with BrdU. BrdU+/Sox2+ cells are seen in the Hensen (G), pillar/tunnel (H), border cell (G and H) and GER (G and H) regions. Multiple Sox2+ mitotic figures (arrows) are present in the GER (G) and two additional mitotic cells (labeled 1 and 2) are shown at higher magnification in the insets. (H) Numerous BrdU+ Claudius cells are detectable. (C, Claudius; D, Deiters; H, Hensen; T, pillar/tunnel; GER, Greater epithelial ridge; WT, wild type). The scale bar in (B) (20 µm) applies to all parts.
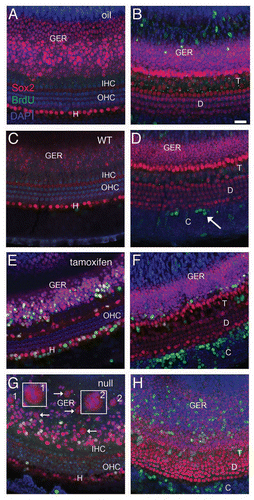
Figure 3 Proliferation in the organ of Corti after induced deletion of p27Kip1 in adult animals. Adult mice were given BrdU ± tamoxifen and observed immediately afterwards (group L6, A–D) or 6 weeks later (group L6-12, E and F). Tissues were immunostained for Sox2 (red) and BrdU (green), with DAPI staining nuclei (blue). Shown are brightest-point projections from a confocal Z-series spanning the sensory epithelium (A, B, E and F) or Deiters' cell nuclear region (C and D, inset for F). No BrdU-labeled cells are detectable in a p27 wild-type (WT) mouse (A), but BrdU+ cells are present in a vehicle-treated adult p27L+/L+;CreER+ animal (B) in the organ of Corti and in the Claudius cell region. (C and D) A tamoxifen-treated p27L+/L+;CreER+ mouse (Group L6) shows two BrdU-labeled Deiters' cells (arrows). The BrdU channel is shown in gray scale in (D). (E and F) Tamoxifen-treated p27L+/L+;CreER+ mouse euthanized 6 weeks later (group L6–12) showing BrdU+ Claudius, Hensen and inner sulcus cells (asterisk). A labeled Deiters' cell (arrow) is also shown in (F) (BrdU channel only in gray scale) and as an inset in a narrower Z-series spanning only the Deiters' nuclei region. All scale bars equal 20 µm. OHC, outer hair cell; IHC, inner hair cell; IS, inner sulcus region; C, Claudius cell region, D, Deiters' cell.
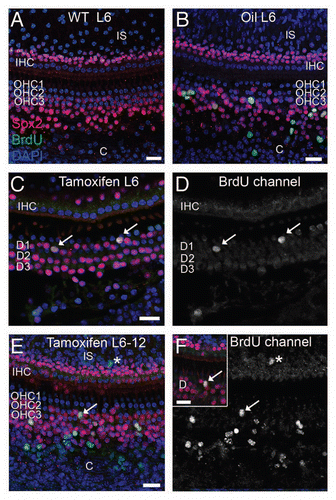
Figure 4 Deletion of p27Kip1 induced in adult p27L+/L+;CreER mice. Dorsal view of pituitaries from p271L+/L+;CreER+ (inducible knockout) mice treated at 15 weeks of age with vehicle (A) or tamoxifen (B) and then examined at 30 weeks of age. Immunostaining for p27Kip1 (red) is apparent in vehicle-treated controls (C), but is eliminated following tamoxifen treatment (D, blue = DAPI nuclear stain). Black scale bar = 1 mm, white scale bar = 100 µm. pd, pars distalis; pi, pars intermedia.
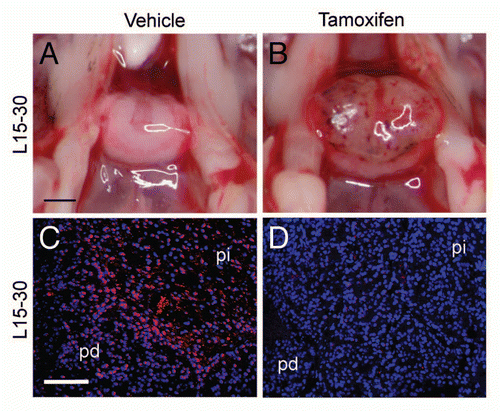
Figure 5 Reactivation of p27Kip1 expression in p27S−/S−;CreER mice. Dorsal view of pituitary (A) from p27S−/S−;CreER+ (inducible knock-on) mice treated with vehicle (A) or tamoxifen (B) at 6 weeks of age and harvested at age 15 weeks (group S6–15). Immunostaining shows absence of p27 in vehicle-treated control pituitary (C), compared to tamoxifen-treated animals (D). Pituitaries from p27S−/S−;CreER+ mice are pathologically enlarged in mice treated with vehicle at 15 weeks of age (E) and observed at 30 weeks of age (group S15–30). Comparable treatment with tamoxifen (F) led to resolution of hemorrhage. An H&E stained section (G) of a tamoxifen-treated mouse pituitary (S15–30 treatment group) shows resolution of hemorrhage and the infiltration of macrophages (m) into Rathke's pouch, which (H) stain positive for CD34 immunoperoxidase (hematoxylin counterstain). Black scale bar = 1 mm (applies to parts A, B, E and F); white scale bar = 100 µm (applies to C, D, G and H). pd, pars distalis; pi, pars intermedia; pn, pars nervosa.
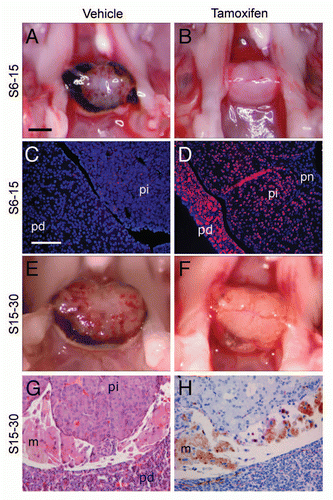
Figure 6 Proliferation and gene expression following loss or reactivation of p27Kip1. Mass of whole pituitaries (A) of p27L+/L+ mice (group L15–30, mean ± SD) treated with tamoxifen (Tam+) and compared to control animals without Cre (CreER−) or treated with vehicle (Tam−). (B) p27S−/S− mice treated at 6 weeks of age (group S6–15) or 15 weeks of age (S15–30) are shown. (C) Cell counts of BrdU-positive cells per high-power field (0.075 mm2, with a mean of 142 ± 12 DAPI+ nuclei) following one week of tamoxifen administration in 6 week-old mice. Homozygous p27loxP mice (L+), p27stop mice (S−) and wild-type (+) mice were examined with or without CreER and tamoxifen. (D) Pars intermedia from p27S−/S−;CreER+ mice treated with IdU and tamoxifen (Tam+) or vehicle (Tam−) followed by CldU and scored for the percentage of IdU, CldU or double-positive cells. (E) RNA expression levels by RT-QPCR are expressed as change in PCR cycle number (−ΔΔCt, mean change ± SEM) for selected genes altered in p27 null pituitary tumors from p27S−/S−;CreER+ mice treated with vehicle vs. tamoxifen. (*p value = 0.01, **p value < 0.001, t-test).
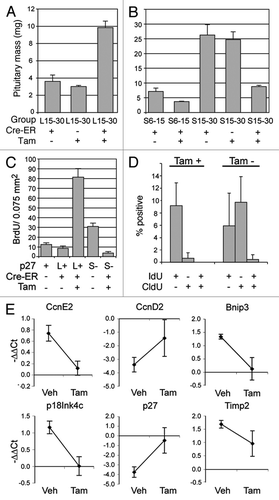
Table 1 Treatment schedules
Table 2 GFP expression in neonatal and adult auditory sensory epithelia in Z/EG; CreER+ reporter miceTable Footnote1
Table 3 BrdU-labeled cell density and retention in neonatal and adult cochleasTable Footnote1