Figures & data
Figure 1 Redox stress induces PKCζ nuclear translocation protecting PKCζ-overexpressing HeLa cells by its own apoptotic effects. (A) PKCζGFP-overexpressing HeLa cells in resting condition (left) and after a 30 min treatment with H2O2 1 mM (right); histograms represent the ratio between the nuclear fluorescence and the cytosolic fluorescence, (184 ± 12.1). (B) Endogenous PKCζ in HeLa cells in resting conditions and after 30 min of treatment with H2O2. (C)(i) Increase dose-dependent in cell viability in PKCζGFP overexpressing HeLa cells: the histograms represents apoptotic counts expressed as difference of % fluorescent cells treated (30 min with different H2O2 concentrations) compared with resting condition (50 µM: 1.7 ± 1.3, 100 µM: 4.9 ± 1.1, 1 mM: 15.7 ± 0.8, 2 mM: 18.5 ± 1.3). (C)(ii) Caspase 3 activity assay: gray line, untreated cells; black line, PKCζGFP-overexpressing cells; dotted-gray line, mock cells treated with H2O2; dotted-black line, PKCζGFP-overexpressing cells treated with H2O2. (D) Apoptotic counts in HeLa cells overexpressing different PKC isoforms (PKCα, PKCε, PKCζ) treated with oxidant agent (H2O2 1 mM) and compared with mock cells (Mock: 1.0 ± 2.1; PKCα −4.7 ± 0.3; PKCε 7.3 ± 0.8; PKCζ 15.7 ± 0.8).
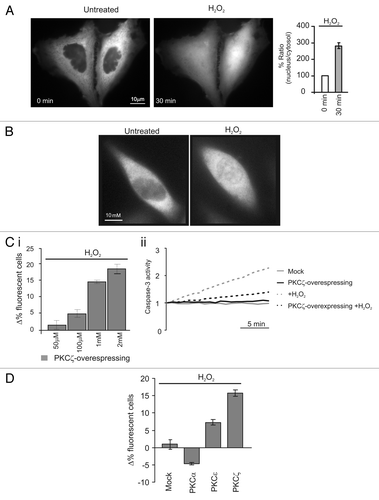
Figure 2 Nuclear PKCζ protects HeLa cells by different apoptotic stimuli. (A) H2O2 (100 µM 30 min) pretreatment induces PKCζ nuclear translocation in PKCζGFP-overexpressing cells, protecting from apoptotic effects of (i) ceramide (30 µM 2 h) and (ii) okadaic acid (0.5 µM 2 h) and (iii) but not from of anti-Fas (4 µg/ml). The upper parts show PKCζGFP-overexpressing HeLa cells following the apoptotic stimulus (left) and following the apoptotic stimulus but previously accompanied by H2O2 100 µM pretreatment (right). The lower parts show the antiapoptotic effects promoted by nuclear translocation of PKCζGFP, mediating H2O2 pre-treatment (cell viability in ceramide-treated cells: Δ% −5.3 ± 1.11 vs. 12.1 ± 0.95 in H2O2 pre-treated HeLa cells; Okadaic acid-treated cells Δ% 2.0 ± 1.21 vs. 14.5 ± 1.23 in H2O2 pre-treated cells; antiFAS-treated cells: Δ% −1.4 ± 1.73 vs. 1.6 ± 1.57 in H2O2 pre-treated HeLa cells). (B) Anti-FAS induces nuclear perturbation as confirmed by nuclear-GFP distribution, where it appears cytosolic-redistributed after 2 h of anti-FAS treatment. (C)(i) LMB (4 µg/ml 2 h) pretreatment induces PKCζ nuclear accumulation in PKCζGFP overexpressing cells, protecting from the apoptotic effects of ceramide (after 2 h of ceramide 30 µM; Δ% cells viability in pre-treated LMB cells: −13.4 ± 7.03 vs. −47.2 ± 8.33 in mock cells. Δ% cells viability in pre-treated LMB cells: −11.0 ± 3.38). (iii) Immunofluorescence of endogenous PKCζ: effect of LMB 4 µg/ml 2 h.
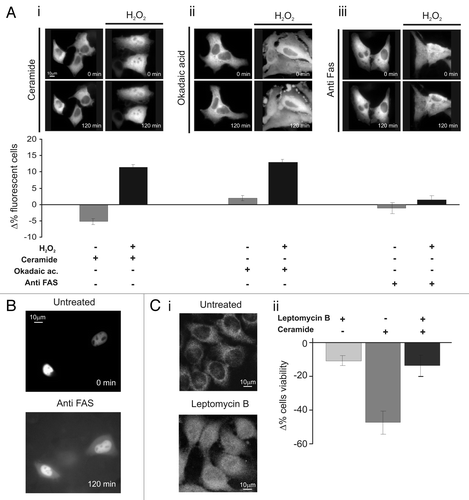
Figure 3 Effects of constitutively nuclear PKCζ (nuPKCζ), constitutively active and constitutively nuclear PKCζ (nuPKCζ-act) and selectively nuclear PKCζ inhibitor (nu-inhPKCζ) on cell viability. (A) Cellular localization and schematic structure of the chimera PKCζ: (i) nuPKCζ and (ii) nuPKCζ-act. (B) Nuclear localization of the different PKCζ chimeras: the histograms represent the ratio between the nuclear and cytosolic average fluorescence intensity, expressed as a percentage, for the three chimera proteins in resting conditions (nuclear distribution of: nuPKCζ-act 4.71 ± 0.80 or nuPKCζ 2.76 ± 0.41 vs. 0.41 ± 0.03 for PKCζ-GFP). (C) Apoptotic counts of HeLa cells expressing the different PKCζ mutants following ceramide treatment (cell viability in nuPKCζ-act and nuPKCζ cells: Δ% 12.6 ± 0.40 or 6.3 ± 1.08 vs. −5.3 ± 1.11 in PKCζGFP expressing HeLa cells, respectively). (D) Cellular localization and schematic structure of the selectively nuclear PKCζ inhibitor (nu-inhPKCζ). (E) Effects of nu-inhPKCζ overexpression on cell viability following LMB pretreatment and/or C2-ceramide treatment (Δ% cells viability in pre-treated LMB cells ceramide untreated: −4.6 ± 2.31 vs. −60.0 ± 8.60 after ceramide treatment or in not LMB pre-treated −48.6 ± 8.71).
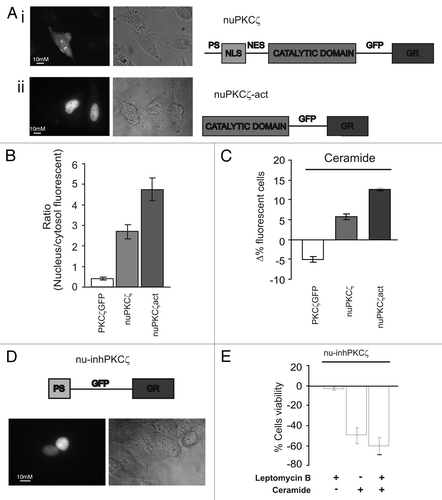
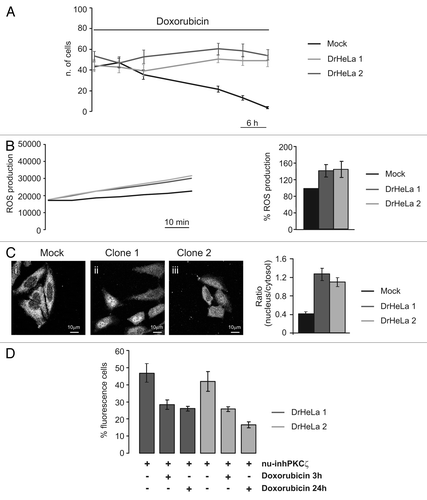
Figure 4 Role of PKCζ nuclear translocation in chemoresistance cells. (A) Doxorubicin resistance of two different clones selected by culturing HeLa cells in presence of Doxorubicin 20 µM. The graph represents a time course of cell viability of three different clones of HeLa cells: a mock clone (black line) and two selected doxorubicin-resistant clones of HeLa cells (gray and dark gray) (Mock: 43.3 ± 4.63, 48.56 ± 4.24, 35.33 ± 4.57, 21.56 ± 3.06, 13.11 ± 2.26, 3.67 ± 0.97; DrHeLa1: 44.25 ± 5.82, 42.75 ± 5.45, 37.50 ± 7.09, 50.75 ± 3.53, 48.88 ± 4.53, 48.88 ± 5.54; DrHeLa2: 56.50 ± 4.42, 48.25 ± 5.58, 55.50 ± 6.61, 63.63 ± 5.48, 61.50 ± 6.31, 56.88 ± 5.03). (B) Time course of ROS production, expressed as normalized values of fluorescence intensity (mock and two doxorubicin-resistant HeLa cells). The histograms represent the increase in ROS production expressed as a percentage respect to mock cells (DrHeLa1 45.1% ± 20.3; DrHeLa2 49.2% ± 25.5). (C) Immunofluorescence of endogenous PKCζ in mock HeLa cells and in two different clones of doxorubicin-resistant HeLa cells. The histograms on the right represent the nuclear distribution of endogenous PKCζ, expressed as ratio between nuclear and cytosolic average fluorescence intensity (mock cells: 0.43 ± 0.04 vs. 1.31 ± 0.16 or 1.11 ± 0.10 in clone 1 or 2, respectively). (D) Apoptotic counts of doxorubicin-resistant HeLa cells transiently expressing nu-inhPKCζ. The histograms represents the difference of percentage of fluorescence following different doxorubicin treatment (20 µM 3 h or 24 h) compared with resting conditions (nu-inhPKCζ expressing DrHeLa1: 46.8 ± 5.23 vs. 28.3 ± 2.75 or 26.1 ± 1.33 after 3 h or 24 h of doxorubicin exposure, respectively; clone2: 41.9 ± 5.66 vs. 25.7 ± 1.45 or 16.6 ± 1.74).