Figures & data
Figure 1 Enforced USP2aWT expression enhanced tumorigenesis in T24 human bladder cancer cells. (A) Proliferation rate in T24 bladder cancer cells transiently engineered to express USP2aWT, T24-USP2aWT and Vec (vector only), T24-Vec. Cells were serum starved for 16 h before changing into growth medium. At the indicated time points, cells were fixed and stained with crystal violet solution. (B) Cell migration capability of T24-USP2aWT and T24 Vec cells were analyzed using gelatin-coated invasion chamber kit (company) as described in the Materials and Methods section. (C) Migration was compared in T24-USP2aWT and T24 Vec cells by a wound-healing assay. (D) T24-USP2aWT and T24-Vec cells were seeded at low density and incubated for colony formation for 7 d. After fixing and staining cells, number of colonies was counted. Representative images were shown. Asterisks in this figure indicate p < 0.05.
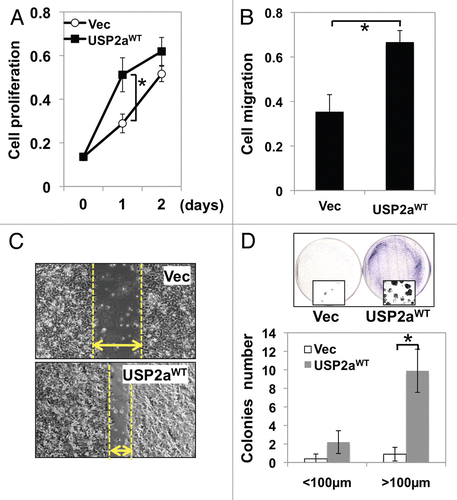
Figure 2 USP2aWT altered activation of signal transduction. (A) Erk/MAPK phosphorylation in USP2aWT expressing T24 cells treated with recombinant HB-EGF (100 ng/ml). Protein from whole-cell lysates at the indicated times were blotted with the Abs against p-Erk/MAPK, Erk/MAPK and β-actin. (B) TUNEL assay showed less apoptotic cells in USP2a-expressing cells compared with control. (C) Levels of cleaved PARP (c-PARP) were detected in whole lysates from T24-USP2aWT and T24-Vec cells incubated in serum free medium (SF), TPA, nocodazole, cisplatin or CHX for 24 h.
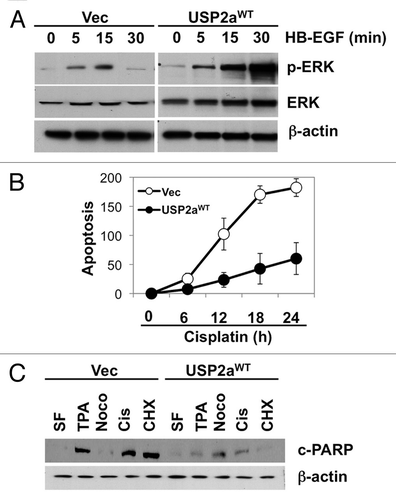
Figure 3 USP2a knockdown by shRNA suppressed oncogenesis. (A) Verification of USP2a gene silencing by stable transfection of T24 cells using two kinds of shUSP2a. USP2a expression level in two engineered cells, shUSP2a-c1 and shUSP2a-c2, was analyzed by western blotting. Control shRNA (shCtrl) was used as negative control. (B) Proliferation of two USP2a-knockdown cell lines was compared with control cells. (C) Wound-healing assay to assess migratory capability of USP2a loss shUSP2a-c2 and control cells. (D) Quantitative analysis using invasion chamber kit of cell migration in USP2a loss cells. (E) USP2a-knockdown cells are more susceptible to apoptotic inducers including a chemotherapeutic drug, cisplatin. Stably engineered cell lines were treated with the indicated reagents (see the Materials and Methods), and protein extracts were applied for western blotting with anti-c-PARP antibody. Asterisks indicate p < 0.05.
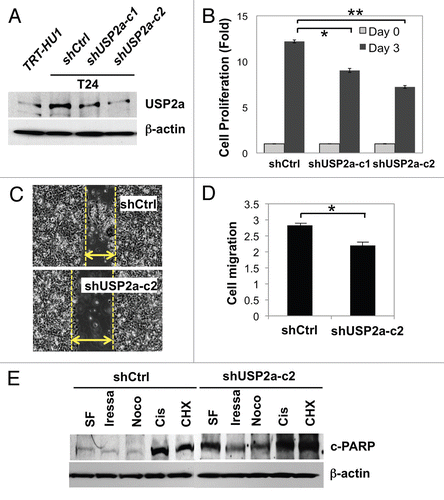
Figure 4 USP2a enhanced the proliferation rate of immortalized human bladder epithelial cells (TRT-HU1) via catalytic activity. (A) A greater colonization by USP2a overexpression in TRT-HU1 cells. Cells were seeded at low density (102/dish) and incubated for 14 d. Colonies were counted after crystal violet staining. (B) Effect of a mutant form of USP2a on proliferation in TRT-HU1 cells. Cell proliferation was evaluated using crystal violet.
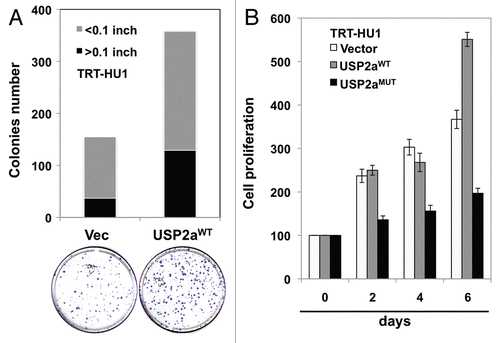
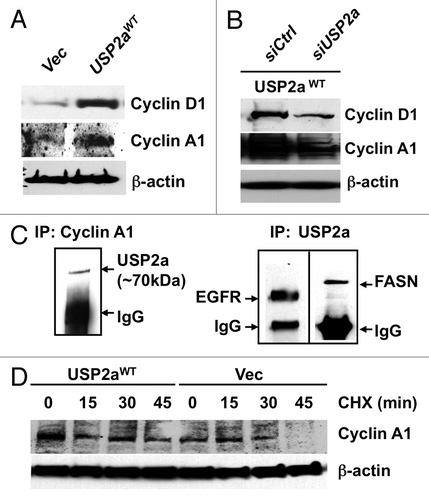
Figure 5 USP2a stabilized cyclin A1. (A) USP2aWT expressing T24 cells expressed higher level of cyclin D1 and cyclin A1. (B) USP2a-targeted oligos, siUSP2a, reversed an increase of cyclin D1 and cyclin A1. (C) Physical interaction of USP2a and cyclin A1. Whole-cell lysates (200 µg) from T24 cells were applied to immunoprecipitation (IP) with anti-cyclin A1 antibody, and IP product was blotted with anti-USP2a antibody (∼70 kDa). The reciprocal co-immunoprecipitation assay was not applied due to no difference between cyclin A1 and IgG (50–55 kDa), although the IP itself was working and we could detect EGFR and FASN co-immunoprecipitated with USP2a. (D) Cyclin A1 degradation was measured in absence or presence of 10 µg/ml CHX in the T24-USP2aWT and T24-Vec cells.