Figures & data
Figure 1 LMW cyclin E overexpression and cyclin E kinase activity in bladder cell lines. (A) Western blot analysis of cyclin E, Cdk2, Cdk4, Cdk6, p27, p21, p16, cyclin D1, p53, Rb, PCNA and actin in bladder cell extracts. (B) Comparison of full-length (FL) and LMW cyclin E protein levels in non-tumorigenic (NT) and tumorigenic (T) bladder cell lines. Mean values ± SEM for NT and T, respectively, were 125.0 ± 15.8 and 176.3 ± 16.8 (p = 0.063) for FL cyclin E and 24.6 ± 3.8 and 90.2 ± 11.6 (p = 0.001) for LMW cyclin E. (C) Immune complexes of Cdk2, p21 and p27 in cell extracts were immunoprecipitated (IP) with polyclonal antibodies to Cdk2, p21 and p27 coupled to protein A-coated beads. Immune complexes were then subjected to western blot (WB) analysis using antibodies to cyclin E, Cdk2, p21 and p27. Normal mammary epithelial cells (N) and breast cancer cells (T) were used as standards. (D) Kinase assays were performed on cell extracts by immunoprecipitation with monoclonal antibodies to cyclin E coupled to protein G-coated beads or polyclonal antibodies to Cdk2, p21 and p27 coupled to protein A-coated beads. Histone H1 was used as the substrate. Lane designations correspond to sample numbers given in .
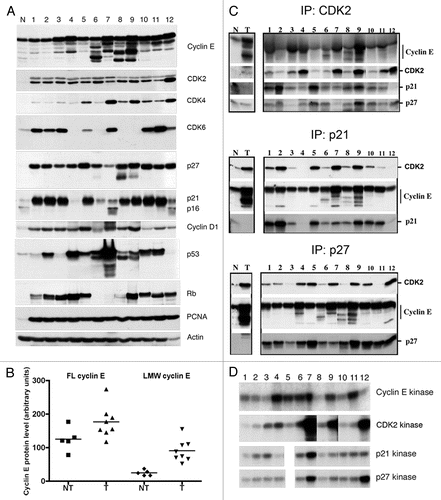
Figure 2 LMW cyclin E protein levels and responsiveness to p27 inhibition in primary tumor samples. (A) Whole-cell lysates were extracted from low-grade (LO) and high-grade (HI) human bladder cancer tissues. Cell extracts were analyzed by western blotting for cyclin E, Cdk2, p27, p21, PCNA and actin. Normal mammary epithelial cells (N) and breast cancer cells (C) were used as standards. (B) Overexpression of LMW cyclin E protein in low- and high-grade tumors. Mean values ± SEM were 39.1 ± 16.1 for grade 2 tumors (n = 10) and 71.6 ± 9.5 for grade 3 tumors (n = 33). (C) Immunoprecipitation (IP) of cyclin E and p27 and western blot (WB) analysis or kinase assays were performed as described in .
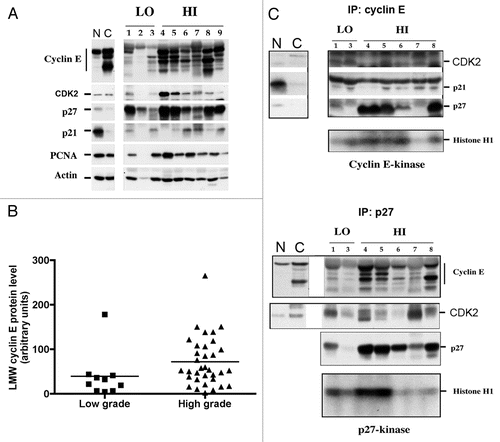
Figure 3 Relationship between high LMW cyclin E protein expression and poor survival in bladder cancer. Bladder tumor specimens from 43 patients were assessed for cyclin E expression by western blot. Patients were stratified by LMW cyclin E expression. Patients with high LMW cyclin E had markedly decreased overall survival (median OS, 30 mo) compared with those with low levels of LMW cyclin E (median OS not reached; p = 0.06).
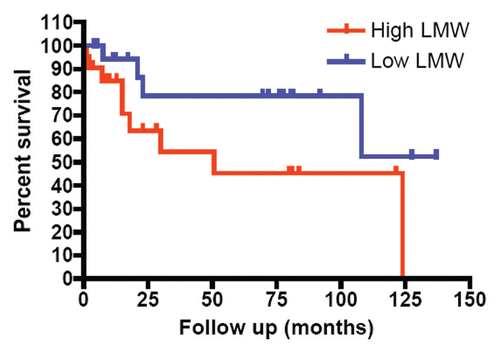
Table 1 Rb, p53, and LMW cyclin E status in five non-tumorigenic and eight tumorigenic bladder cell lines
Table 2 Patient characteristics and tumor sample results
Table 3 Association between expression of LMW cyclin E, p21 and p27 protein and pathologic features of primary bladder cancers