Figures & data
Figure 1. Tetraploidization is suppressed by CHK1 and p53. (A) Chk1, p53 and p21 proteins were assessed in derivatives of DLD-1 by immunoblot. α-tubulin was probed as a loading control. (B) Representative images of diploid (left) and tetraploid (right) metaphase chromosome spreads. (C) Representative images of chromosome 18 signals. Nuclei were counterstained with DAPI (blue). (D) The number of chromosome 18-specific FISH signals per nucleus was assessed in wild type DLD-1 cells (CHK1+/+; n=550) and in CHK1-haploinsufficient derivatives (CHK1+/-; n=350). (E) Mitotic cells stained with antibodies to γ-tubulin (red) to detect centrosomes and α-tubulin (green) to detect mitotic spindles. Bipolar (left) and multipolar spindles (right) were observed. Nuclei were counterstained with DAPI. (F) Chk1, p53 and α-tubulin (loading control) proteins were assessed in derivatives of HCT116. Bars,10 μm.
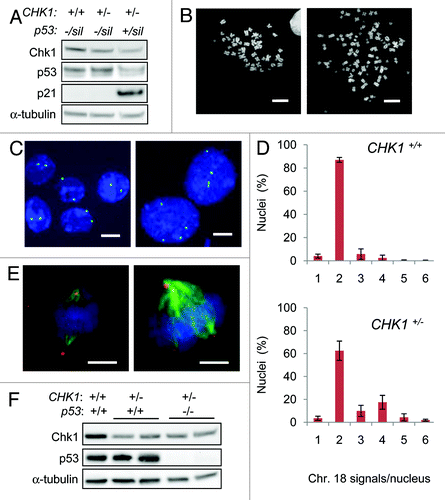
Table 1. Analysis of metaphase spreads.
Figure 2. CHK1-haploinsufficient, p53-mutant human cells bypass mitosis and undergo whole genome endoreduplication. (A) Sequential images of CHK1+/+ and CHK1+/- DLD-1 cells expressing a histone H2B-GFP fusion protein were captured by time lapse fluorescence microscopy. Black arrows indicate cells undergoing mitosis. White arrows track cells that fail to condense chromatin during the experiment. The fates of histone H2B-GFP expressing cells tracked over 72 h were scored (n > 90). (B) Incorporation of EdU in asynchronous cells or in cells synchronized in S-phase. (C) Representative image of diplochromosomes found in metaphase spreads of CHK1+/- cells. Bar, 10 μm.
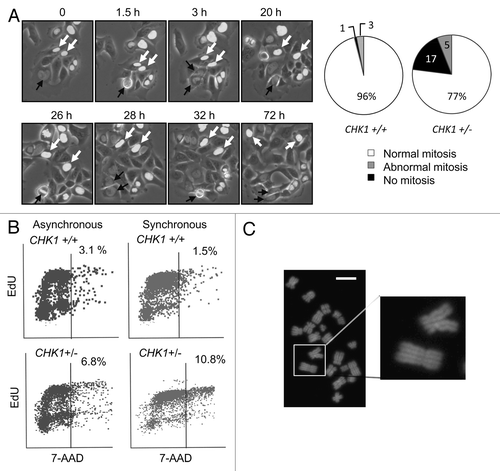
Figure 3. Reduction in CHK1 gene dosage causes reduction in Cdk1 activity. (A) CHK1+/+ and CHK1+/- DLD-1 cells harboring mutant p53 were synchronized by double thymidine block, released, and harvested at the indicated time points. Proteins immunoprecipitated from non-denatured lysates with an anti-Cdk1 antibody were assessed for histone H1 kinase activity. 32P-labeled histone H1 was resolved by gel electrophoresis and assessed by autoradiography and scintillation counting. Total H1 protein was visualized by staining with Coomassie blue. (B) Chk1 and Cdk1 total protein, and Cdk1-Y15-P and H3-S10-P phosphoproteins were assessed in lysates from cells synchronized as in (A), by immunoblot. α-tubulin was assessed as a loading control. (C) Hemaglutinin (HA) epitope-tagged Cdk1 (Cdk1-HA) was stably expressed in pooled clones derived from CHK1+/- cells. The expression of exogenous Cdk1 protein was assessed by immunoblotting with an anti-HA antibody; endogenous and exogenous Cdk1 protein (indicated with an asterisk) were probed with an anti-Cdk1 antibody. Ploidy was assessed by numerical analysis of metaphase chromosomes (n = 50). Con, empty vector control.
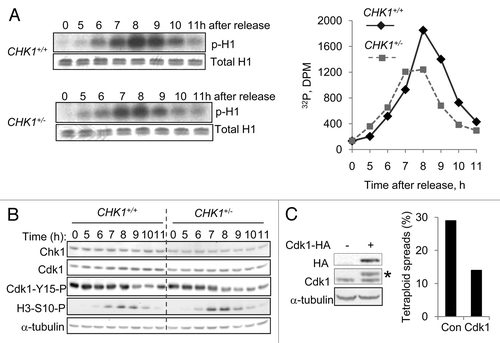
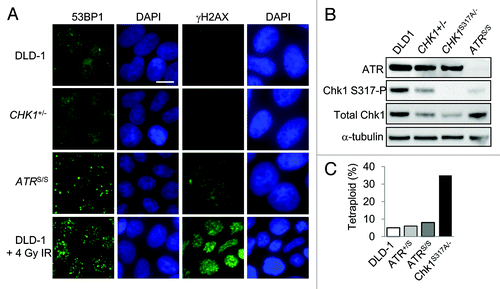
Figure 4. DNA damage and tetraploidization in p53-mutant cells with altered ATR-Chk1 signaling. (A) The localization and distribution of 53BP1 and γH2AX were assessed by immunofluorescence in untreated DLD-1 and derivatives with the indicated genotypes. Where indicated, cells were fixed 90 minutes after treatment with 4 Gy ionizing radiation. Nuclei were counterstained with DAPI. Bars, 20 um. (B) Levels of ATR, Chk1 and Chk1 S317-P phosphoprotein in cells treated with 10 Gy ionizing radiation were determined by immunoblot. (C) Ploidy was assessed in DLD-1 and the indicated derivatives by numerical analysis of metaphase chromosomes (n > 20).