Figures & data
Figure 1. Rad9Ser272 is phosphorylated in an ATM-dependent manner. (A) Rad9Ser272 is phosphorylated in response to DNA damage. Rad9 becomes phosphorylated at Ser272 in response to IR, UV, CPT (6 h) and HU (1 h) and phosphorylation is inhibited by an ATM specific inhibitor KU55933 (10 µM) (left panel) and ATM specific siRNA (right panel). (B) Rad9Ser272 phosphorylation is not affected in ATR-Seckel cells after damage.
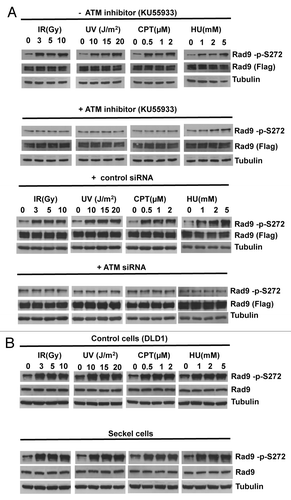
Figure 2. Rad9Ser272 is phosphorylated in a MRN complex-dependent manner. Damage-induced Ser272 phosphorylation is inhibited by siRNAs of MRE11, Rad50 and Nbs1.
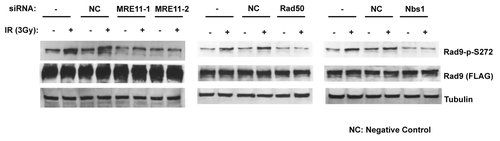
Figure 3. ATM-dependent Rad9 phosphorylation at Ser272 is important to control recombination pathways. The Rad9-S272C/A mutant cells show decreased homologous recombinations induced by I-SCEI, p <0.01 (student’s t-test).
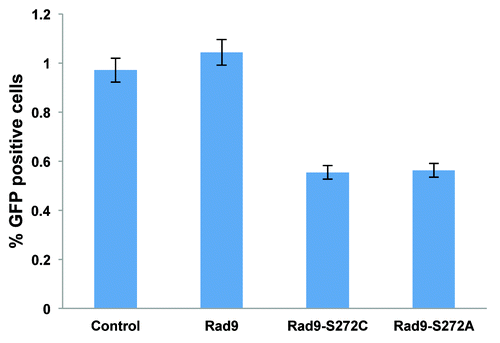
Figure 4. The Rad9-S272C mutant cells accumulate DNA damage. (A) Rad51 foci formation in Rad9 and Rad9-S272C cells before and after IR (3 Gy). (B) Timing of Rad9Ser272, H2AX, Chk1 and Chk2 phosphorylation in response to IR (3 Gy).
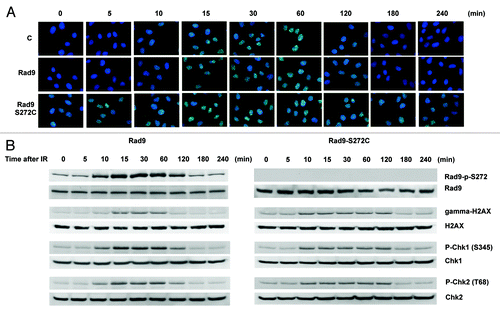
Figure 5. Rad9Ser272 is phosphorylated during normal cell cycle in an ATM dependent manner. (A) Cell cycle progression and Rad9S272 phosphorylation after double thymidine block with (right) and without KU55933 treatment (10 µM) (left). (B) Rad9Ser272 phosphorylation in sorted cells with (right) and without KU55933 (10 µM) treatment (left).
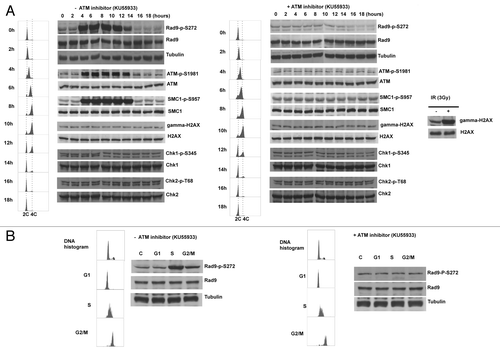
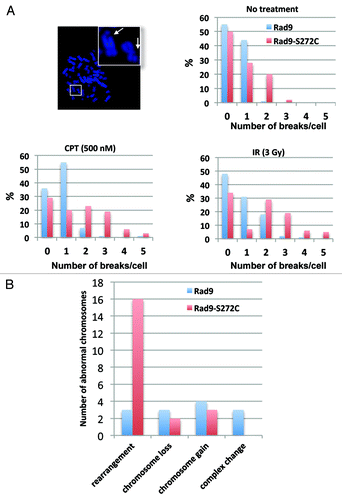
Figure 6. The Rad9-S272C mutant cells accumulate chromosome breakages and show chromosome instability phenotypes. (A) Chromosome breakages in Rad9 and Rad9-S272C cells. An example of metaphase spread of a Rad9-S272C cell (top, left). The Rad9-S272C cells induce a high frequency of chromosome breakages without damage (top, right), after IR treatment (3 Gy) (bottom, right), after CPT treatment (500nM, 6 h) (bottom, left). (B) The Rad9-S272C mutant cells induce a high frequency of GCRs, chromosome rearrangements. Twenty cells for each cell line were karyotyped and chromosome abnormalities were detected.