Figures & data
Figure 1. Canonical TGFβ signaling. TβRII phosphorylates TβRI in response to TGFβ binding. Activated TβRI phosphorylates Smad3 at its C-terminus, which releases it from the anchoring protein SARA and permits formation of a Smad3-Smad4 complex. This heteromeric complex then translocates to the nucleus, associates with DNA-binding co-activators and co-repressors and regulates the expression of target genes. I-Smads antagonize TGFβ signaling by preventing phosphorylation of Smad3 and thus act as a negative feedback mechanism. PPM1A is a phosphatase that can dephosphorylate Smad3 phosphorylated at its C-terminal SXS motif.
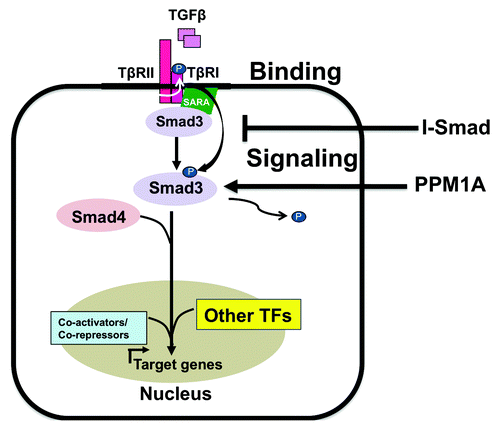
Figure 2. TGFβ/Smad3 signaling and the cell cycle. CyclinD/CDK4/6 and Cyclin E/CDK2 mediate the transition of the cell cycle from the G1 to S phase by phosphorylating Rb and thus preventing it from sequestering E2F. E2F is then free to drive the transcription of G1/S phase transition proteins. TGFβ/Smad3 signaling promotes cell cycle arrest through its induction of p15 and p21, in conjunction with Sp1 and repression of c-myc, in conjunction with E2F4/5 and p107. Ras, in cooperation with Pin1, can increase the transcriptional activity of cyclin D, inducing tumor promotion.
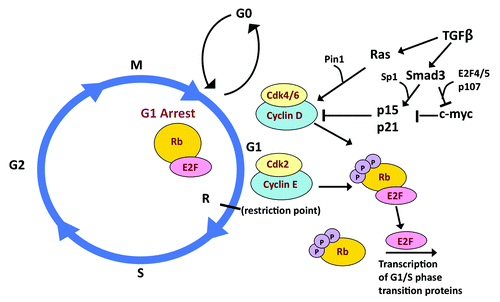
Figure 3. Smad3 phosphorylation sites. Smad3 is phosphorylated by TβRI at the C-terminus and by various cancer-associated kinases at indicated sites. The MH1 domain is responsible for DNA binding, while the MH2 domain mediates interactions with co-activators and co-repressors.
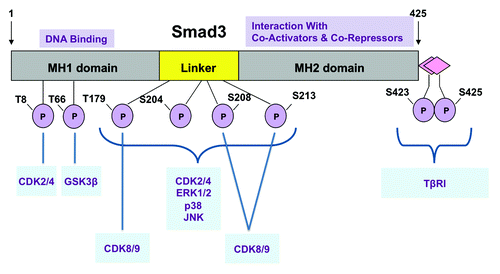
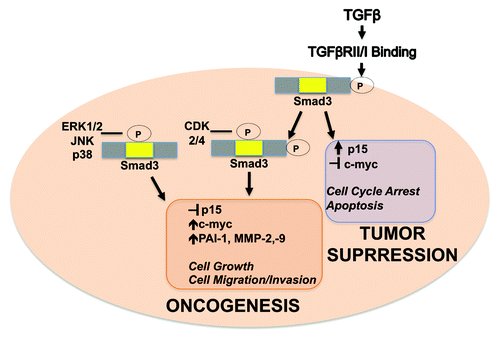
Figure 4. Model for dichotomous Smad3 signaling. Differential phosphorylation of Smad3 results in the induction of TGFβ-mediated tumor suppressive genes in early-stage breast cancer cells or oncogenic genes in late-stage breast cancer cells. Nuclear kinases CDKs 2/4 phosphorylate Smad3 after pSmad3C translocates into the nucleus, while cytoplasmic MAPKs can phosphorylate Smad3 inducing nuclear activation independent of TGβRI phosphorylation.