Figures & data
Figure 1. In yeast cultured under CR conditions on 0.2% glucose, there are two critical periods when the addition of LCA to growth medium can extend longevity. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Chronological lifespan analysis was performed as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 8–12). Abbreviations, D, diauxic; L, logarithmic; PD, post-diauxic; ST, stationary phase.
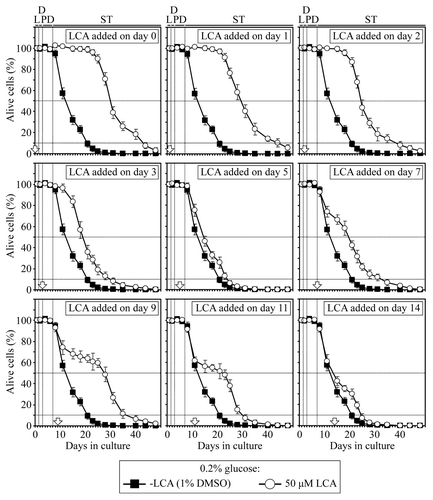
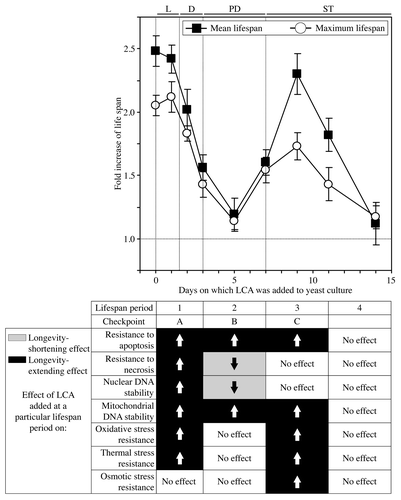
Figure 3. In yeast cultured under CR conditions on 0.2% glucose, there are two critical periods when the addition of LCA to growth medium can extend longevity. In yeast cultivated under non-CR conditions on 2% glucose, there is only one such a period. The mean (A) and maximum (B) chronological lifespans of different wild-type yeast cultures are shown. Wild-type yeast cells were cultured under CR or non-CR conditions and LCA was added as described in the legends for and , respectively. Chronological lifespan analysis was performed as described in “Materials and Methods.” Data are presented as mean ± SEM [n = 8–12 for (A); n = 6–7 for (B)]; *p < 0.05 (relative to the mean or maximum chronological lifespan of yeast not exposed to LCA).
![Figure 3. In yeast cultured under CR conditions on 0.2% glucose, there are two critical periods when the addition of LCA to growth medium can extend longevity. In yeast cultivated under non-CR conditions on 2% glucose, there is only one such a period. The mean (A) and maximum (B) chronological lifespans of different wild-type yeast cultures are shown. Wild-type yeast cells were cultured under CR or non-CR conditions and LCA was added as described in the legends for Figures 1 and 2, respectively. Chronological lifespan analysis was performed as described in “Materials and Methods.” Data are presented as mean ± SEM [n = 8–12 for (A); n = 6–7 for (B)]; *p < 0.05 (relative to the mean or maximum chronological lifespan of yeast not exposed to LCA).](/cms/asset/ac415b2f-61e5-4ea0-8fe6-d16e7868e326/kccy_a_10921754_f0002.gif)
Figure 2. In yeast cultured under non-CR conditions on 2% glucose, there is only one critical period when the addition of LCA to growth medium can extend longevity. Wild-type yeast cells were cultured in YP medium initially containing 2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Chronological lifespan analysis was performed as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 6–7).
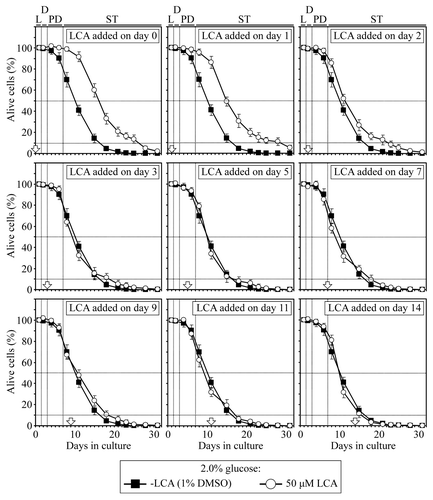
Figure 4. There are several ways through which LCA could differentially influence some longevity-extending and longevity-shortening processes following its addition at different periods of yeast chronological lifespan. LCA could control these longevity-defining processes at certain checkpoints that may exist in L/D and early ST phases (periods 1 and 3, respectively). PD and late ST phases constitute periods 2 and 4, respectively. See text for details.
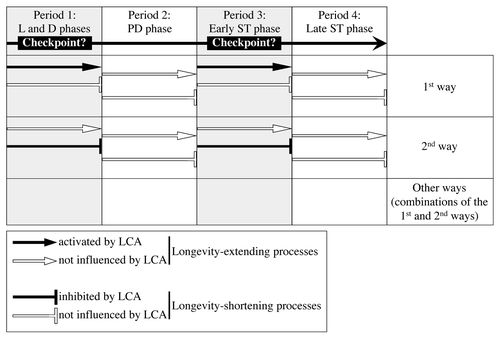
Figure 5. LCA reduces the susceptibility of yeast cells to mitochondria-controlled apoptotic death, a longevity-shortening process, only if added at period 1, 2 or 3 of chronological lifespan and for the rest of the lifespan. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Cell viability assay for monitoring the susceptibility of yeast to an apoptotic mode of cell death induced by a 2 h exposure to exogenous hydrogen peroxide was performed as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 3–5); *p < 0.05 (relative to the % of alive cells in yeast cultures not exposed to LCA).
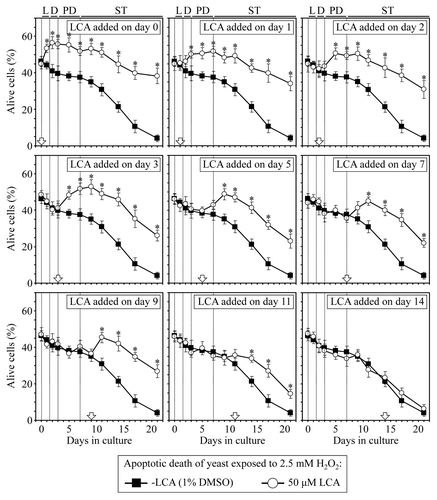
Figure 6. LCA differentially influences the susceptibility of chronologically aging yeast to palmitoleic acid-induced necrotic cell death, a longevity-shortening process, if added at different periods of lifespan. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Cell viability assay for monitoring the susceptibility of yeast to a necrotic mode of cell death induced by a 2 h exposure to exogenous palmitoleic acid was performed as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 4); *p < 0.05 (relative to the % of alive cells in yeast cultures not exposed to LCA).
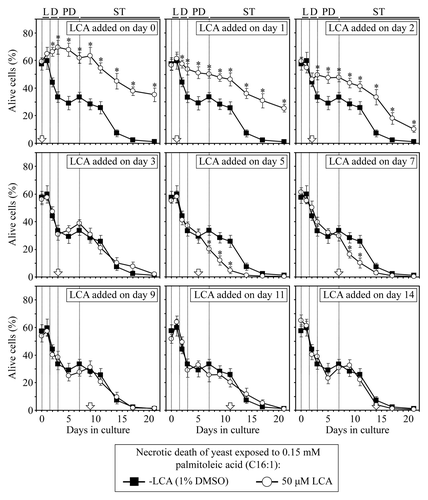
Figure 7. LCA differentially influences the maintenance of nDNA integrity, an essential longevity-extending process, if added at different periods of yeast lifespan. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). The frequency of spontaneous point mutations in the CAN1 gene of nDNA was measured as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 3); *p < 0.05 (relative to the frequency of spontaneous point mutations in the CAN1 gene of nDNA in yeast cultures not exposed to LCA).
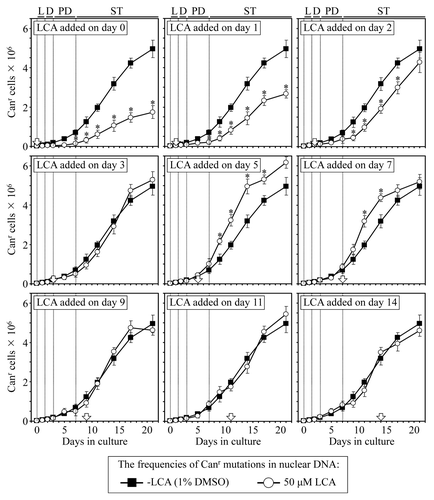
Figure 8. LCA differentially influences the maintenance of mtDNA integrity, an essential longevity-extending process, if added at different periods of yeast lifespan. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). The frequencies of spontaneous single-gene (mit- and syn-) and deletion (rho- and rhoo) mutations in mtDNA, all causing a deficiency in mitochondrial respiration and impairing growth on glycerol, were measured as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 5–6); *p < 0.05 (relative to the frequencies of spontaneous mit-, syn-, rho- and rhoo mutations in mtDNA in yeast cultures not exposed to LCA).
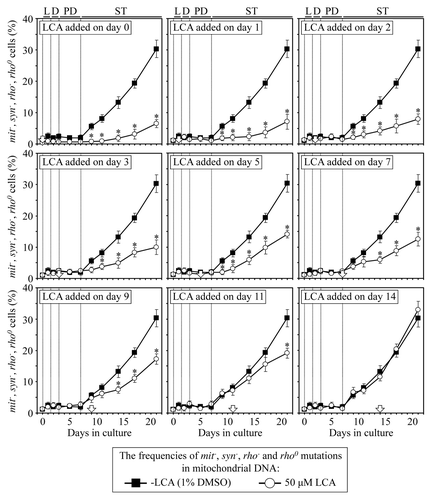
Figure 9. LCA differentially influences the maintenance of mtDNA integrity, an essential longevity-extending process, if added at different periods of yeast lifespan. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). The frequencies of spontaneous point mutations in the rib2 and rib3 loci of mtDNA were measured as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 3–4); *p < 0.05 (relative to the frequencies of spontaneous rib2 and rib3 mutations in mtDNA in yeast cultures not exposed to LCA).
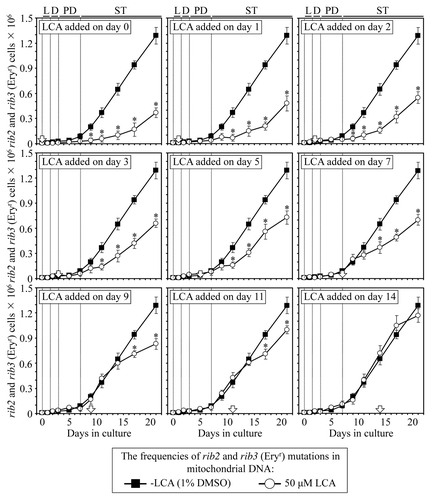
Figure 10. Effect of LCA added at different periods of chronological lifespan on the ability of yeast to resist chronic oxidative stress. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Spot assays for monitoring oxidative stress resistance were performed as described in “Materials and Methods.” Serial 10-fold dilutions of cells were spotted on plates with solid YP medium containing 2% glucose as carbon source and 5 mM hydrogen peroxide. All pictures were taken after a 3-d incubation at 30°C.
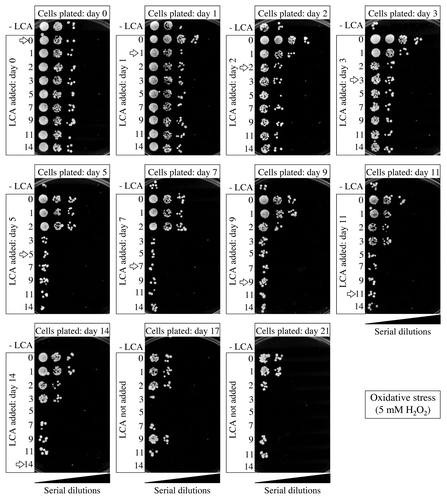
Figure 11. Effect of LCA added at different periods of chronological lifespan on the ability of yeast to resist chronic thermal stress. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Spot assays for monitoring thermal stress resistance were performed as described in “Materials and Methods.” Serial 10-fold dilutions of cells were spotted on plates with solid YP medium containing 2% glucose as carbon source. Plates were initially incubated at 55°C for 30 min, and were then transferred to 30°C. All pictures were taken after a 3-d incubation at 30°C.

Figure 12. Effect of LCA added at different periods of chronological lifespan on the ability of yeast to resist chronic osmotic stress. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Spot assays for monitoring osmotic stress resistance were performed as described in “Materials and Methods.” Serial 10-fold dilutions of cells were spotted on plates with solid YP medium containing 2% glucose as carbon source and 1 M sorbitol. All pictures were taken after a 3-d incubation at 30°C.

Figure 13. A mechanism that may link the ability of LCA to extend longevity of CR yeast only if added at period 1 or 3 of chronological lifespan to the differential effects of this compound on certain longevity-extending and longevity-shortening processes controlled at different lifespan periods. LCA may control these longevity-defining processes at the checkpoints A, B and C that exist in L/D (period 1), PD (period 2) and early ST (period 3) phases, respectively. See text for details.