Figures & data
Figure 1. ORCA is polyubiquitinated in vivo. (A) Immunoblot analysis of U2OS whole-cell lysates using antibodies against ORCA. The arrowhead indicates unmodified ORCA, and the bar indicates the high molecular weight smear. The asterisk denotes the cross-reacting band. (B) Immunoblot analysis of the whole-cell lysates of U2OS cells transfected with T7-tagged ORCA (+) or control vector (-) using antibodies against T7. Note the high molecular weight smear in (+). (C) Flag immunoprecipitation of U2OS cells co-expressing T7-ORCA and Flag-Ub. Note the polyubiquitinated T7-ORCA as denoted by the high molecular weight smear. Cells only transfected with T7-ORCA were used as the immunoprecipitation control. Note that the arrowhead likely denotes the ORCA that has at least one ubiquitin attached, since it was immunoprecipitated-using FLAG in cells expressing FLAG-Ub. (D) Affinity pull-down in cells expressing T7-ORCA and varying amount of 6 × His-Ub (0, 1, 2 µg). Polyubiquitinated T7-ORCA was pulled down by His tag. (E) HA immunoprecipitation of U2OS cells co-expressing HA-ORCA and Flag-Ub under the denaturing condition (see Materials and Methods). Mouse IgG immunoprecipitation serves as the control.
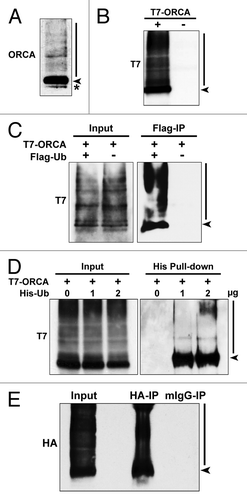
Figure 2. Ubiquitination of ORCA is elevated at the G1/S boundary. U2OS cells were synchronized at G1 or G1/S boundary and treated with DMSO or MG132 for 6 h. Whole-cell lysates were analyzed by immunoblots using antibodies against ORCA. Note the accumulation of the high molecular weight smear in lane four (the MG132-treated sample compared with DMSO at the G1/S boundary). Tubulin serves as the loading control. The arrowhead represents unmodified ORCA and the asterisk represents the cross-reacting band.
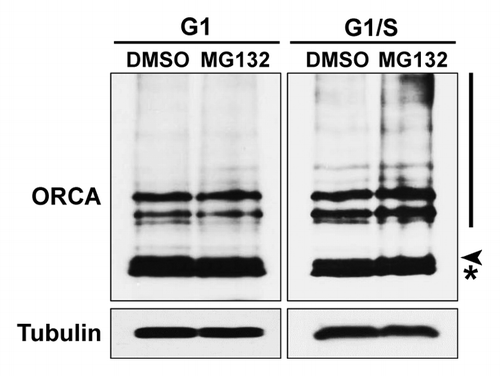
Figure 3. ORCA ubiquitination occurs on multiple lysine residues in the WD domain. (A) Schematic representation of various truncation mutants of ORCA (adapted and modified from ref. Citation29). T7 epitope was constructed at the N terminus. (B) T7 immunoprecipitation of the ORCA mutants followed by T7 immunoblot analysis. Note that only the WD-containing mutants and the full-length protein display the high molecular weight smear. (C) Schematic representation of all the 14 lysine residues within the WD domain that were mutated into arginine residues. Individual point mutations, several combined mutations and the mutants having all the lysines mutated (T7-ORCA.K-R or T7-ORCA.270–647.K-R) were tested for ubiquitination. (D) Immunoblot analysis of cell extracts expressing T7-vector control, T7-ORCA and T7-ORCA.K-R using T7 antibody. (E) Immunoblot analysis of cell extracts expressing T7-vector control, T7-ORCA.270–647 and T7-ORCA.270–647.K-R using T7 antibody.
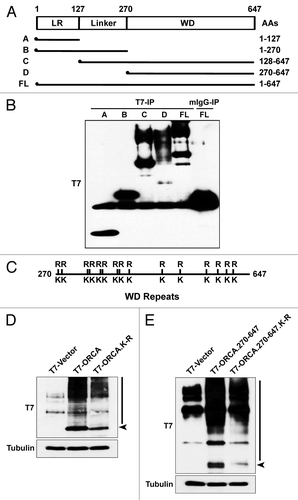
Figure 4. ORCA polyubiquitin chain is formed through lysine-48 of the ubiquitin. (A) Immunoblot analysis of the whole-cell lysates of U2OS cells transfected with T7 control vector or T7-tagged ORCA using antibodies against K48 ubiquitin. Short, non-saturated exposure shows the high molecular weight smear in T7-ORCA-expressing cells. (B) Immunoblot analysis of the whole-cell lysates of U2OS cells transfected with T7 control vector or T7-tagged ORCA [with (+) or without (-) MG132 treatment] using antibodies against K48 ubiquitin. Note the accumulation of the high molecular weight smear upon MG132 treatment. Long, saturated exposure shows the presence of K48 ubiquitin in all the samples (including controls). (C and D) HA immunoprecipitations in U2OS cells expressing HA-ORCA with (+) or without (-) Flag-Ub. Immunoblot analysis using K48-specific (C) or K63-specific (D) antibodies. Note the polyubiquitinated HA-ORCA is in the form of K-48 linkage.
![Figure 4. ORCA polyubiquitin chain is formed through lysine-48 of the ubiquitin. (A) Immunoblot analysis of the whole-cell lysates of U2OS cells transfected with T7 control vector or T7-tagged ORCA using antibodies against K48 ubiquitin. Short, non-saturated exposure shows the high molecular weight smear in T7-ORCA-expressing cells. (B) Immunoblot analysis of the whole-cell lysates of U2OS cells transfected with T7 control vector or T7-tagged ORCA [with (+) or without (-) MG132 treatment] using antibodies against K48 ubiquitin. Note the accumulation of the high molecular weight smear upon MG132 treatment. Long, saturated exposure shows the presence of K48 ubiquitin in all the samples (including controls). (C and D) HA immunoprecipitations in U2OS cells expressing HA-ORCA with (+) or without (-) Flag-Ub. Immunoblot analysis using K48-specific (C) or K63-specific (D) antibodies. Note the polyubiquitinated HA-ORCA is in the form of K-48 linkage.](/cms/asset/3347c4b8-2560-4f63-b054-48a39a28113c/kccy_a_10921870_f0004.gif)
Figure 5. Orc2 prevents ORCA from proteasome-mediated degradation. (A) Immunoblot analysis of whole-cell extracts from cells treated with Orc2 siRNAs in the absence (-) or presence (+) of proteasomal inhibitor MG132. Varying concentrations (25%, 50% and 100%) of whole-cell extracts treated with luciferase siRNAs (control) are shown to provide information on the percentage of knockdown and reduction of protein levels. Note the destabilization of ORCA and Orc3 in Orc2-depleted U2OS cells and the inhibition of ORCA degradation in the presence of MG132. Orc3 is destabilized upon Orc2 depletion but cannot be stabilized upon MG132 treatment, whereas Cdt1 protein levels are elevated upon MG132 treatment. (B) Depletion of Orc2 in human WI38 cells. Note the degradation of ORCA and Orc3 in Orc2-depleted cells, but stabilization of ORCA, as well as evidence of polyubiquitinated ORCA in the presence of MG132. (C) Depletion of ORCA and Orc2 in YFP-ORCA-expressing cells. Immunofluorescence analysis was performed using Trf2 antibody. The scale bar represents 30 µm. (D) Immunoprecipitation using Orc2, T7 or mouse IgG antibody in T7-ORCA-expressing cells. Note Orc2 associates with non-ubiquitinated ORCA. The arrowhead in unbound fractions denotes the absence of non-ubiquitinated form of ORCA in the Orc2 IP unbound sample. Note the enrichment of ubiquitinated forms of ORCA in the Orc2 IP unbound sample. (E) Immunoprecipitation using HA antibody in cells co-expressing HA-Orc2 and T7-ORCA.
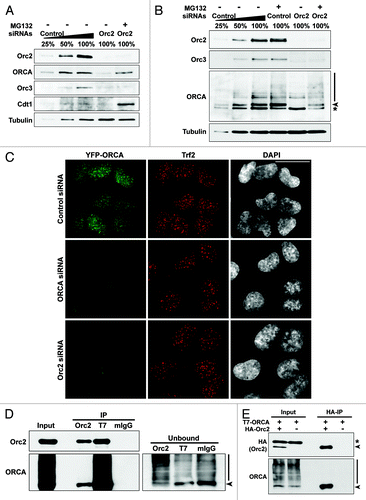
Figure 6. The polyubiquitinated ORCA associates with detergent-resistant chromatin fraction. (A) YFP-ORCA-expressing U2OS cells were incubated with DMSO and MG132, and were then fixed directly by paraformaldehyde (PF) or pre-extracted with detergent before fixation (PE) to remove soluble proteins. Note that YFP-ORCA localized in the form of aggregated foci upon MG132 treatment, which are resistant to detergent extraction. (B) Immunostaining of DMSO and MG132-treated YFP-ORCA-expressing cells with K48 ubiquitin antibody. (C) Immunostaining of DMSO and MG132-treated YFP-ORCA-expressing cells with Fibrillarin shows that the ORCA-containing foci do not localize to the nucleolar structures in the cells. (D) Biochemical fractionation of YFP-ORCA expressing cells treated with DMSO or MG132 followed by immunoblot analysis using GFP antibody. (S) represents cytosolic fraction, and (P) denotes chromatin-bound fraction. Note the high molecular weight smear of YFP-ORCA predominantly on the chromatin fraction (P) upon MG132 treatment. (E) Snapshots of the FRAP analyses on DMSO or MG132-treated YFP-ORCA-expressing cells. The insets denote where the photobleach was conducted. (F) Quantitations of FRAP data show a highly dynamic rate of ORCA on chromatin, with the recovery time T(1/2) = 0.30 ± 0.07s to a 0.93 ± 0.04 recovery fraction, whereas, in MG132-treated cells, no more than 20% recovery after photobleach was observed. Scale bars in (A, B, C and E) represent 10 µm.
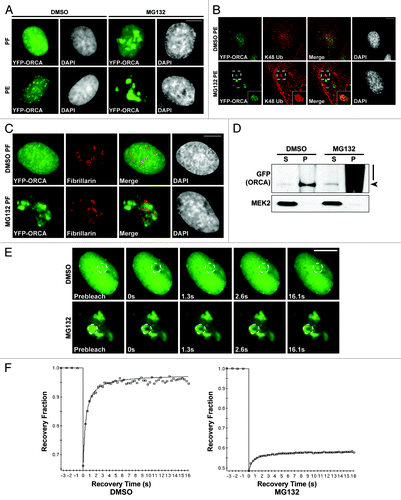
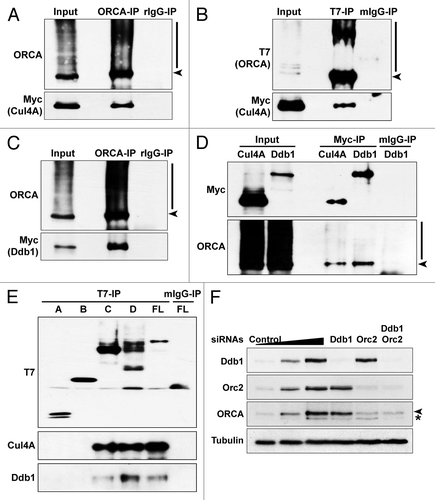
Figure 7. ORCA is associated with Cul4A-Ddb1 E3 ligase. (A) ORCA immunoprecipitation in T7-ORCA and Myc-Cul4A transfected cells followed by immunoblots with ORCA and Myc. Rabbit IgG serves as the control. (B) T7 immunoprecipitation in cells transfected with T7-ORCA and Myc-Cul4A followed by immunoblots with T7 and Myc. Note that ORCA associates with Cul4A. Mouse IgG serves as the control. (C) ORCA immunoprecipitation in cells transfected with T7-ORCA and Myc-Ddb1 followed by immunoblots with ORCA and Myc. Rabbit IgG serves as the control. (D) Myc immunoprecipitations in cells transfected with T7-ORCA and Myc-Cul4A or Myc-Ddb1 followed by immunoblots with Myc and ORCA. Mouse IgG serves as the control. (E) Immunoprecipitations in cells expressing various T7-ORCA mutants and Myc-Cul4A were performed using the T7 antibody, and Cul4A and the endogenous Ddb1 were analyzed by immunoblots. Note that only the WD-containing constructs interact with Cul4A-Ddb1. (F) Depletion of Ddb1 or in combination with Orc2 followed by immunoblot analysis of ORCA.