Figures & data
Figure 1. HER2-Akt signaling regulates Aurora B stability. (A) HER2 overexpression positively regulates the steady-state expression of CSN6. Indicated equal amounts of cell lysates were immunoblotted with indicated antibodies. (B) Akt positively regulates the steady-state expression of CSN6. 293T cells were co-transfected with indicated plasmids and increasing amounts of Akt. Equal amounts of cell lysates were immunoblotted with indicated antibodies. (C) Akt-overexpressing cells have increased steady-state expression of CSN6. Indicated amounts of cell lysates were immunoblotted with indicated antibodies. (D) Antagonizing Akt leads to destabilization of CSN6 in HER2-overexpressing cells. Indicated amounts of cell lysates were immunoblotted with indicated antibodies. (E) Dmominant Akt reduces the expression levels of CSN6. Indicated amounts of cell lysates were immunoblotted with indicated antibodies. (F) Inhibitor of PI3K-Akt pathway causes destabilization of CSN6. Rat1-Akt cells were treated with LY294002 for 6 h before harvesting. Equal amounts of cell lysates were immunoblotted with indicated antibodies.
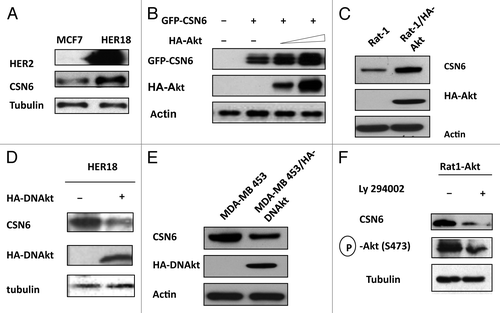
Figure 2. Akt induces CSN6 nuclear import. (A) CSN6 nuclear localization is induced in the presence of Akt. U2OS cells were transfected with indicated plasmids. Nucleus was stained with Hoechst 33342 and pseudo colored with red. (B) CSN6 is localized at the nucleus at Akt-overexpressing cells. Indicated cells were stained with Anti-Csn6 followed with FITC-conjugated secondary Ab. Nucleus was stained with DAPI.
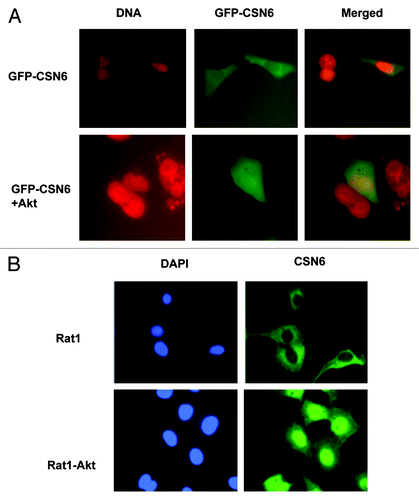
Figure 3. HER2-Akt signaling reduces CSN6 turnover via decreasing CSN6 ubiquitination. (A) CSN6 turnover rate is decreased in HER2-overexpressing cells. Indicated cells were treated with cycloheximide (CHX) (100 µg/ml) for the indicated times. Cell lysates were immunoblotted with indicated antibodies. (B) Akt dereases the turnover of CSN6. Indicated 293T cells were transfected with GFP-CSN6 with or without Akt and then treated with cycloheximide (CHX) (100 µg/ml) for the indicated times. (C) CSN6 turnover rate is increased in Akt activity deficient cells. Indicated cells were treated with cycloheximide (CHX) (100 µg/ml) for the indicated times. Cell lysates were immunoblotted with indicated antibodies. (D) Akt stabilizes CSN6 by decreasing CSN6 ubiquitination. 293T cells were transfected with the indicated plasmids. The proteasome inhibitor MG132 was added 6 h before harvesting cells. The ubiquitinated amount of CSN6 is analyzed by immunoprecipitated (IP) with anti-GFP followed by immunoblotting (IB) with anti-ubi.
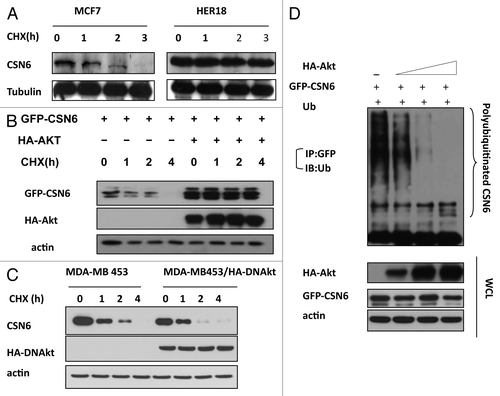
Figure 4. Akt interacts with and phosphorylates CSN6. (A) Interaction of Akt with CSN6. Equal amounts of 293T cell lysates transfected with indicated plasmids were immunoprecipitated (IP) with anti-Flag and immunoblotted (IB) with anti-HA. Equal amounts of U2OS cell lysates were also immunoprecipitated with anti-CSN6 and immunoblotted with anti-Akt. (B) Consensus phosphorylation sequence for Akt phosphorylation sites in CSN6. (C) CSN6 is a phosphorylated target of Akt. 293T cells were transfected with the indicated expression vectors in the presence or absence of LY294002. Equal amounts of cell lysates were immunoprecipitated (IP) with anti-Flag antibody and immunoblotted with anti-phosphorylated Akt substrates antibody. (D) Akt phosporylates CSN6 in vitro. Baculovirus-produced active recombinant Akt1 was incubated with indicated equal amounts of bacterially produced and purified recombinant CSN6 and mutant CSN6 (S60A) protein for kinase activity. 32P autoradiography demonstrated the phosphorylated CSN6 and Coomassie blue-stained SDS-PAGE gel of CSN6 proteins showed comparable amounts of wild-type CSN6 and mutant CSN6 (S60A).
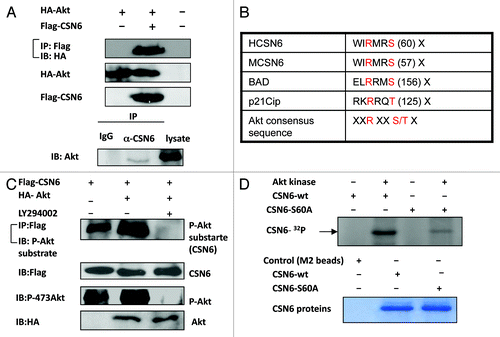
Figure 5. CSN6 phosphorylation mutant S60A has different stability. (A) The steady-state expression of phosphorylation mutant CSN6S60A. 293T cells were co-transfected with indicated plasmids and increasing amounts of Akt. Equal amounts of cell lysates were immunoblotted with indicated antibodies. (B) Turnover rate of CSN6 phosphorylation mutant S60A is accelerated. Indicated wt and CSN6 mutant tranfected cells were treated with cycloheximide (CHX) (100 µg/ml) for the indicated times. Cell lysates were immunoblotted with indicated antibodies. (C) Akt upregulates CSN6 by decreasing CSN6 ubiquitination. 293T cells were transfected with the indicated plasmids. The proteasome inhibitor MG132 was added 6 h before harvesting cells. The ubiquitinated amount of CSN6 is analyzed by immunoprecipitated (IP) with anti-myc followed by immunoblotting (IB) with anti-HA. (D) Akt reduces CSN6 ubiquitination in the presence of FBXW7. 293T cells were transfected with the indicated plasmids. The proteasome inhibitor MG132 was added 6 h before harvesting cells. The ubiquitinated amount of CSN6 is analyzed by immunoprecipitated (IP) with anti-GFP followed by immunoblotting (IB) with anti-ubi.
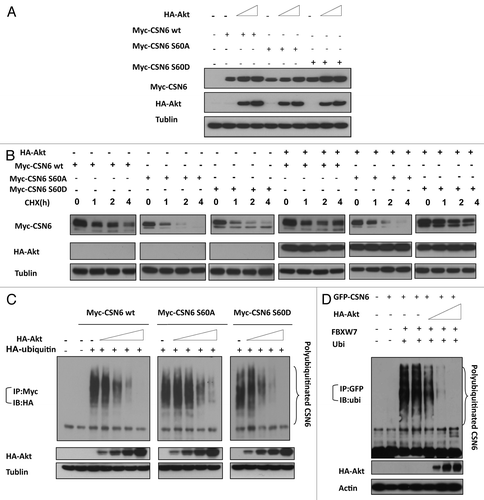
Figure 6A-C. CSN6 S60D mutant efficiently induces p53 degradation and enhances transformation. (A) CSN6 reduces the levels of MDM2 ubiquitination. 293T cells were transfected with the indicated plasmids. The proteasome inhibitor MG132 was added 6 h before harvesting cells. The ubiquitinated amount of MDM2 is analyzed by immunoprecipitated (IP) with anti-MDM2 followed by immunoblotting (IB) with anti-ubi. (B) CSN6 S60D mutant efficiently degrades p53. A549 cells were transfected with indicated plasmids and equal amounts of cell lysates were were immunoblotted with indicated antibodies. (C) Akt enhances CSN6-mediated cell transformation. Cells were transfected with indicated plasmids, and foci-formation assay was performed.
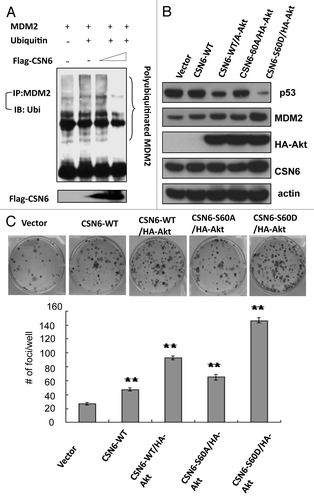
Figure 6D. (D) Enrichment score graph and Ranked list metric graph showing the upregulation of p53-suppressed target genes in CSN6-highly expressing patients (Gene Set Enrichment Analysis, Broad Institute, MIT. Cohort: GSE-20194 consisting of 255 untreated stage I–stage III breast cancer patients at MD Anderson Cancer Center. Blue, p53-suppressed target genes; red, p53-activated target genes.
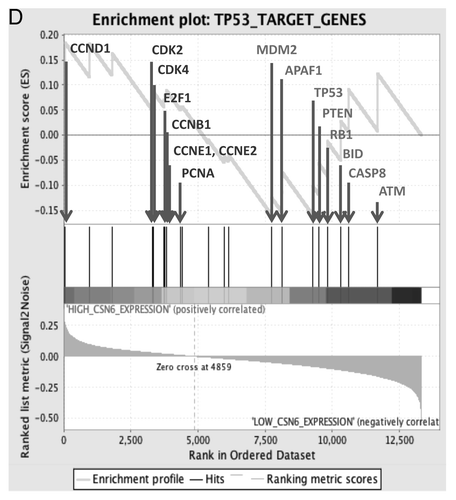
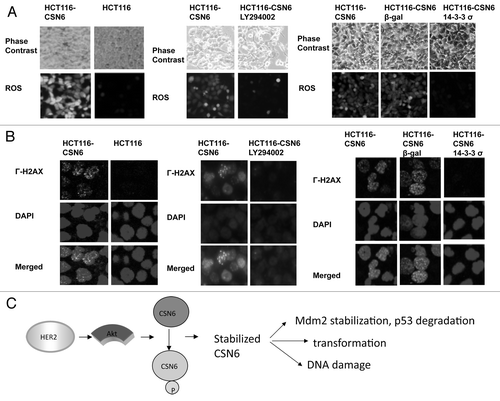
Figure 7. Impacts of Akt-CSN6 axis on ROS production and DNA damage. (A) ROS production (green fluorescence) was detected by DCFDA and fluorescence microscopy in indicated cells. Phase-contrast images and merged images of the same microscopic fields are shown. (B) The distribution of γ-H2AX foci (green) was analyzed in indicated cells by immunofluorescent microscopy with anti-γ-H2AX and Alexa Fluor 488-conjugated secondary antibodies. DNA was counterstained with DAPI (blue). (C) Model of the impact of the Akt-CSN6 axis in regulating p53, cell transformation and DNA damage.