Figures & data
Table 1. Transcripts of proteins involved in mitochondrial biogenesis and mitochondrial protein translation are upregulated in human breast cancer cells as compared with adjacent stromal cells
Table 2. Transcripts encoding the mitochondrial ATP synthase are upregulated in human breast cancer cells, as compared with adjacent stromal cells
Table 3. Transcripts encoding proteins associated with ketone body re-utilization are upregulated in human breast cancer cells, as compared with adjacent stromal cells
Figure 1. AKAP1, a mitochondrial marker, is predominantly confined to epithelial cancer cells, and largely absent from adjacent stromal cells, in human breast cancer tissues. Paraffin-embedded sections of human breast cancer tumor tissue were immunostained with antibodies directed against AKAP1. Slides were then counter-stained with hematoxylin. Note that AKAP1 is highly expressed in the epithelial compartment (brown color). Two representative images are shown. Original magnification is 60x, as indicated.
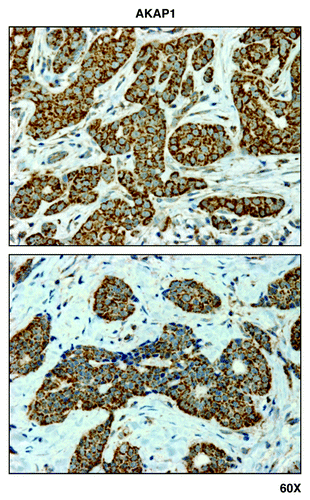
Figure 7. MCT1, a metabolic marker for the uptake of high-energy mitochondrial fuels, is predominantly localized to epithelial cancer cells, and absent from adjacent tumor stromal cells, in human breast cancers. Paraffin-embedded sections of human breast cancer tumor tissue were immunostained with antibodies directed against MCT1. Slides were then counter-stained with hematoxylin. Note that MCT1 immunostaining is largely absent from the stromal compartment and confined to the epithelial compartment (brown color). Original magnification is 60x, as indicated.
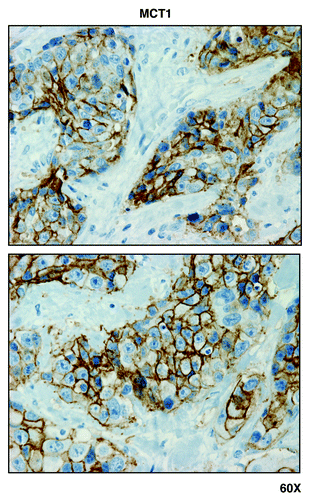
Figure 2. GOLPH3 and GOLPH3L, markers of mitochondrial lipid biosynthesis, are localized mainly to epithelial cancer cells in human breast cancer tissues. Paraffin-embedded sections of human breast cancer tumor tissue were immunostained with antibodies directed against GOLPH3 and GOLPH3L. Slides were then counter-stained with hematoxylin. Note that both GOLPH3 family members are largely absent from the stromal compartment and confined to the epithelial compartment (brown color). Original magnification is 40x, as indicated.
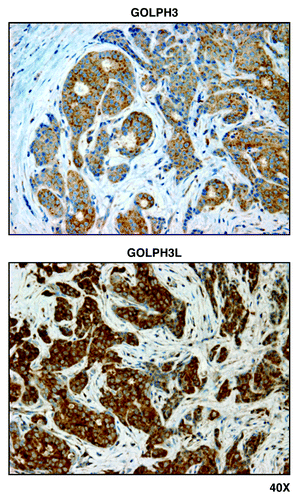
Figure 3. Mitochondrial ribosomal proteins (MRPL40, MRPS7, MRPS15, and MRPS22) are localized to epithelial cancer cells, but absent from adjacent tumor stroma, in human breast cancers. Paraffin-embedded sections of human breast cancer primary tumors were immunostained with antibodies directed against MRPL40, MRPS7, MRPS15, and MRPS22 (all mitochondrial ribosomal proteins). Note that immunostaining (brown color) is largely confined to the epithelial cancer cells. Original magnification, 40x.
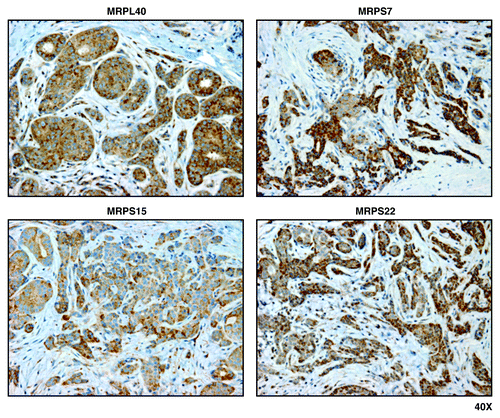
Figure 4. PGC1-α, a key mitochondrial transcription factor, is largely confined to epithelial cancer cells, and absent from stromal cells, in human breast cancers. Paraffin-embedded sections of human breast cancer primary tumors were immunostained with antibodies directed against PGC1-α. Note that PGC1-α immunostaining is largely confined to the epithelial cancer cells. A red arrow points at an area that is further magnified below and is shown as an inset. Original magnification, 60x.
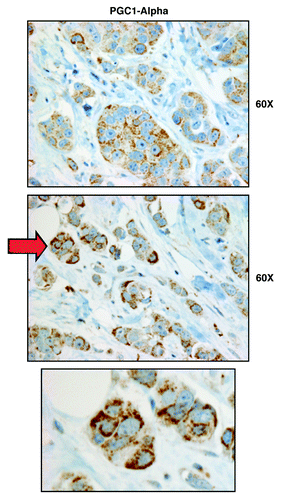
Figure 5. NRF1 and NRF2 family members preferentially label epithelial cancer cells in human breast cancers, but not adjacent stromal cells. Paraffin-embedded sections of human breast cancer primary tumors were immunostained with antibodies directed against either NRF1 (panel A) or NRF2 (panel B). Note that NRF1/2 immunostaining is largely confined to the epithelial cancer cells. Original magnification is as indicated.
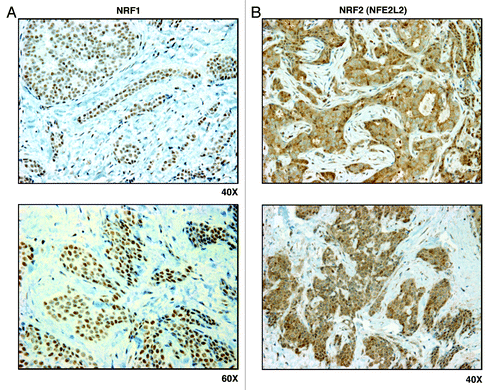
Figure 6. Markers of mitochondrial biogenesis (TFAM, POLRMT, TOMM70A, and TIMM9) are all predominantly confined to epithelial cancer cells in human breast cancer tumor tissues, but are largely absent from adjacent stromal cells. Paraffin-embedded sections of human breast cancer tumor tissue were immunostained with antibodies directed against TFAM, POLRMT, TOMM70A and TIMM9. Slides were then counter-stained with hematoxylin. Note that TFAM, POLRMT, TOMM70A and TIMM9 are all largely absent from the stromal compartment and confined to the epithelial compartment (brown color). Original magnifications, 40x and 60x, are as indicated.
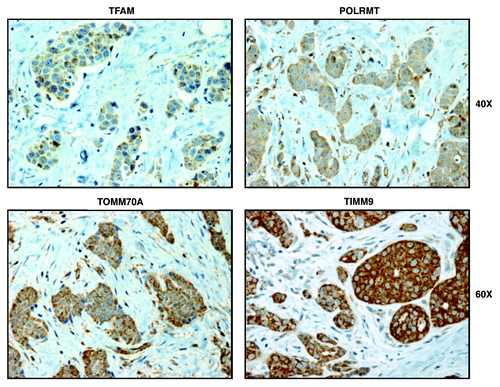
Figure 8. GOLPH3 (a marker of mitochondrial lipid synthesis) and POLRMT (a marker of mitochondrial translation) both predict poor clinical outcome in human breast cancer patients. Note that the expression levels of the gene transcripts for GOLPH3 (A) and POLRMT (B) predict poor overall survival, especially in ER-positive (A) patients. Numbers of cases with annotation are shown. P values are as indicated. X-Tile software was employed to identify subpopulation cut-points to observe maximum survival differences between the high expression and low expression subpopulations. The Log-rank test was used to evaluate the significance of differences in survival curves for high vs. low expressing populations.
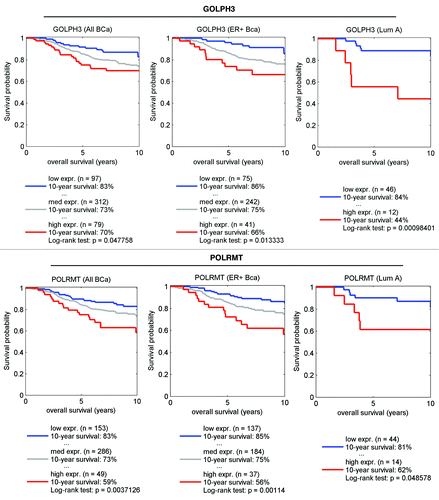
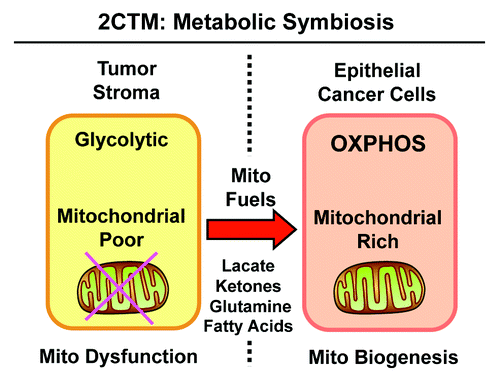
Figure 9. Two-compartment tumor metabolism (2CTM) reflects metabolic symbiosis. We suggest that aggressive breast cancers consist of two distinct metabolic compartments. In the tumor microenvironment, stromal fibroblasts (and other cell types) show signs of mitochondrial dysfunction, are mitochondrial-deficient, and metabolically shift toward aerobic glycolysis (the “reverse Warburg effect”). This results in the stromal production of high-energy mitochondrial fuels, such as L-lactate, ketone bodies, glutamine and free fatty acids. These recycled nutrients are then available to “feed” neighboring cancer cells. In response to this energy-rich microenvironment, epithelial cancer cells undergo mitochondrial biogenesis, amplifying their capacity for oxidative mitochondrial metabolism (OXPHOS). Thus, the tumor stroma and epithelial breast cancer cells are metabolically linked in a “symbiotic/parasitic” relationship, related to energy transfer or an energy imbalance.