Figures & data
Figure 1. Overexpression of Nek1 leads to pVHL degradation. (A) HEK cells were transfected with Nek1-pcDNA3.1-Myc/His or empty vector to collect whole-cell lysate 36 h later for immunoblot analysis of pVHL, Nek1-Myc/His and β-actin. (B) Densitometric analysis of immunoblots. pVHL signal was normalized by β-actin signal in the immunblots from three experiments. (C) HEK cells were transfected with Nek1-pcDNA3.1-Myc/His or empty vector for 36 h and then treated with 100 nm Bortezomib for 4 h to collect whole-cell lysate for immunoblot analysis of VHL, Nek1-Myc/His and β-actin.
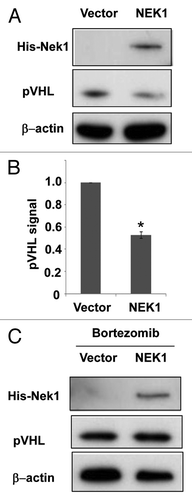
Figure 2. Nek1 interacts with and phosphorylates pVHL. (A) HEK 293 cells were co transfected with Nek1-pcDNA3.1-Myc/His and HA-VHL-pRc/CMV to collect whole-cell lysate 36 h for immunoprecipitation using anti-HA antibody, followed by immunoblot analysis using anti-Myc and anti-HA antibodies. (B) HEK cells were transfected with Nek1-pcDNA3.1-Myc/His or empty vector to collect cell lysate 36 h later for immunoprecipitation of Nek1-Myc/His. The immunoprecipitates were incubated with recombinant pVHL in the presence of (γ-32P)ATP. The samples were then resolved by gel electrophoresis, followed by autoradiography. (C) Purified recombinant active Nek1 and pVHL were incubated separately or together in kinase reaction buffer with (γ-32P)ATP. The samples were then resolved by gel electrophoresis, followed by autoradiography. (D) purified recombinant Nek1 and pVHL were incubated in kinase reaction buffer, followed by gel electrophoresis and Commassie Blue staining. The pVHL band was exercised for mass spectrometry analysis of phosphorylated sites (highlighted in red at indicated sites).
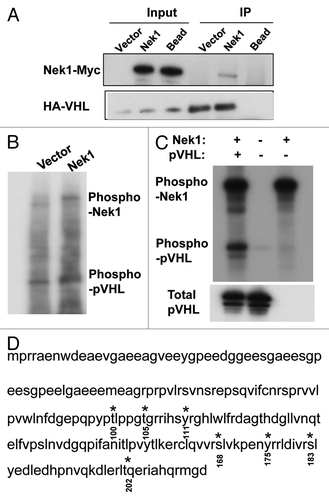
Figure 3. Mutation of pVHL at S-168 increases protein stability. HEK cells were transfected with HA-pVHL or its mutants for 36 h. The cells were then treated with cycloheximide to collect lysates at indicated time points for immunoblot analysis of HA-pVHL (A) or its mutants (B–D) using anti-HA antibodies. The results of densitometry are shown in (E).
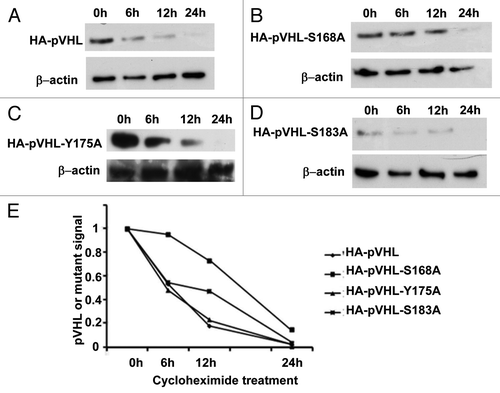
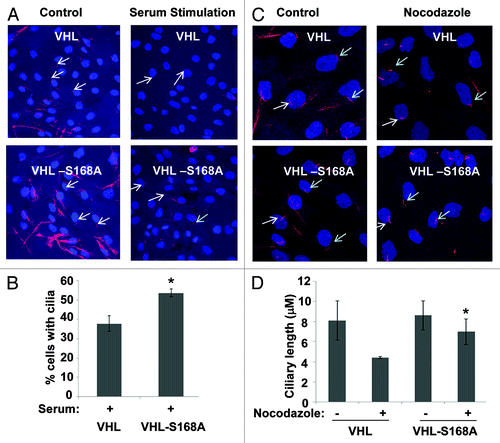
Figure 4. pVHL-S168A induces cilia that are more stable than those induced by pVHL. RCC4 cells were stably transfected with VHL or its S168A mutant. The cells were serum-starved for 48 h, followed by 24 h of serum stimulation or 3 h of nocodazole treatment. The cells were fixed for acetyl-tubulin immunofluorescence to evaluate cilia. (A and C) Representative images. Red, acetyl-tubulin staining; blue, nuclei: arrows, representative cilia. (B) Cilia were counted to determine the percentage of cells with cilia after serum stimulation. Data: mean+/−SD, n = 3; * p < 0.05 vs. pVHL-reconstituted cells after serum stimulation. (D) The length of cilia was measured using LSM image analyzer. Data: mean+/−SD, n = 20; * p < 0.01 vs. pVHL-reconstituted cells after nocodazole treatment.