Figures & data
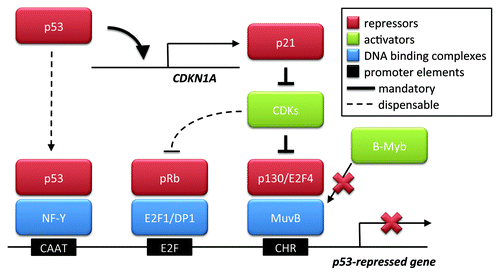
Figure 1. p53-mediated gene repression. p53 reportedly can act as a repressor of genes by at least three mechanisms. The first one involves the association of p53 with the transcription factor complex NF-Y, which, in turn, binds the CAAT box of promoter DNA. The two other mechanisms each depend on the transactivation of p21 by p53. p21 blocks cyclin-dependent kinases (CDKs), leading to the hypophosphorylation of retinoblastoma (Rb) family members. These then associate with E2F proteins. E2Fs can bind their cognate DNA elements, in cooperation with DP1, and the associated Rb proteins then mediate repression. However, p130, while binding E2F4 but independent of an E2F-binding DNA element, associates with the MuvB-complex, replacing B-Myb. MuvB binds the CHR element of DNA. As a consequence of E2F4 and p130 being tethered to the CHR, the promoter is repressed. The first two mechanisms were reported earlier, but the article in this issue of Cell CycleCitation1 argues against a direct association of p53 with the cyclin B promoter; nor did the authors observe a need for E2F binding sites in repression. Only the last mechanism, driven by MuvB and the CHR element, appears indispensable for gene repression in these experiments. This mandatory mechanism is therefore indicated by bold arrows in the scheme, whereas the other two seem dispensable, reflected by dashed lines.