Figures & data
Figure 1. A flowchart describing the derivation of acquired radioresistant 31FR-31NR cells. Cells were exposed to FR consisting of a 0.5 Gy X-ray fraction dose at every 12 h, 6 d a week. The cells treated with this exposure scenario with 62 fractions for 31 d were referred to as 31FR cells. The 31FR cells were further cultured without irradiation for more than 31 d and the resulting cells were designated as 31FR-31NR cells.
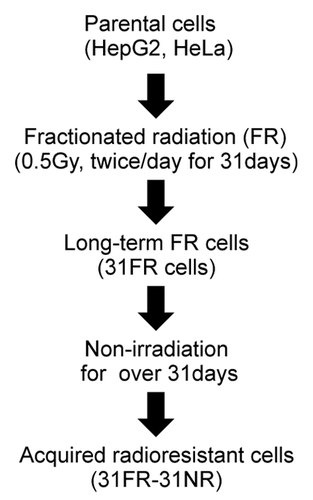
Figure 2. Results for neutral comet assays. (A) Distributions of tail moment values for 0FR and 31FR-31NR cells. Results for HepG2 and HeLa cells are shown in the left and right panels, respectively. The median tail moment values are indicated at the bottom of the graph. (B) Distributions of tail moment values for 0FR and 31FR-31NR cells derived from the HeLa cell line. Samples were prepared at 48 h after transfection with control siRNA (siControl) or cyclin D1 siRNA (siCyclin D1). Median tail moment values are indicated at the bottom of the graph.
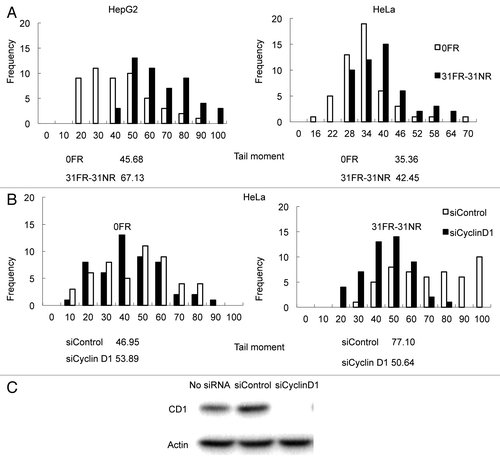
Figure 3. Cdk4-independent DSBs formation in 31FR-31NR cells (A) Cell cycle distributions for HeLa cells with either control siRNA (siControl) or Cdk4 siRNA (siCdk4). Percentages of G1-, G2/M- and S-phase cells are shown with the standard deviations in parentheses in the lower panel. (B) Western blotting results for Cdk4, γ-H2AX and actin in 0FR and 31FR-31NR cells derived from HeLa cell line. Cell extracts were prepared at 48 h after transfection with either control siRNA or Cdk4 siRNA. (C) Western blotting results for γ-H2AX and actin in the 0FR and 31FR-31NR cells derived from HeLa cell line. The cells were treated with 1.9 μM Cdk4-I for 24 h.
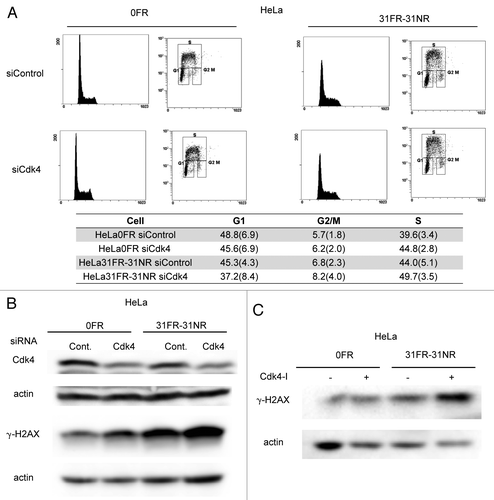
Figure 4. Cyclin D1-mediated slowing down of replication forks. (A) Image of red- and green-labeled DNA tracks. Replication of DNA in 0FR cells is shown by the arrow. Short-labeled DNA tracks in 31FR-31NR cells are shown by arrowheads. (B) Histograms of labeled DNA lengths in 0FR and 31FR-31NR cells. Results for HepG2 and HeLa cells are shown in the left and right panels, respectively. The average lengths are indicated at the bottom of the graph. (C) Histograms of labeled DNA lengths in the HepG2 and HeLa cells that expressed wild-type cyclin D1(CD1-WT) or cyclin D1 mutants (CD1-T286A).
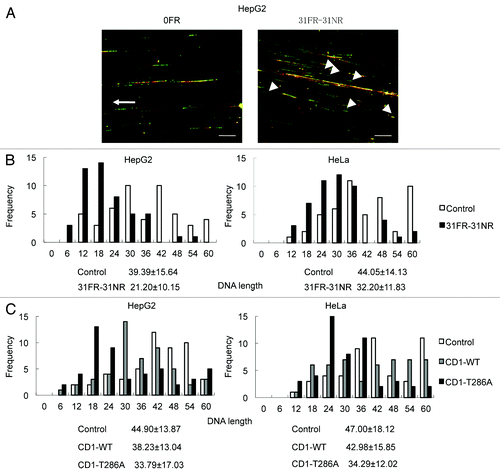
Figure 5. Mus81-mediated DSBs in 31FR-31NR cells. (A) Western blotting results for Mus81, γ-H2AX and actin in 0FR and 31FR-31NR cells derived from HepG2 cell line. Cell extracts were prepared at 24 h after transfection with either control siRNA (Cont.) or Mus81 siRNA. The amounts of γ-H2AX were normalized by corresponding actin level. The values are expressed relative to the control value of 0FR cells with control siRNA. (B) Cyclin D1 (green) and γ-H2AX (red) in 0FR cells and 31FR-31NR cells of HeLa are shown. Immunofluoresence were performed 48 h after transfection with control siRNA or Mus81 siRNA. Double-staining cells with cyclin D1 and γ-H2AX are indicated by arrows.
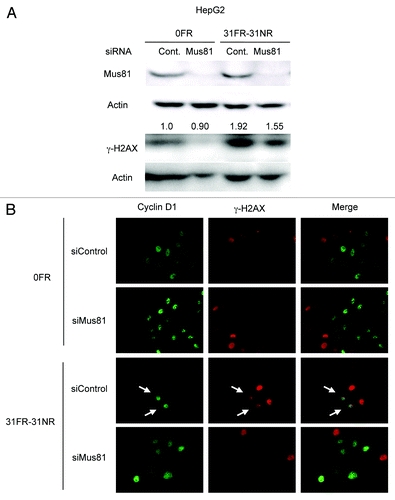
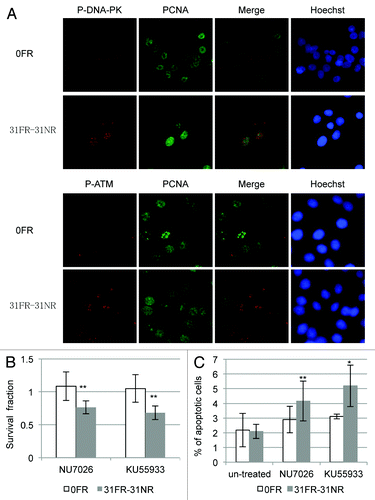
Figure 6. DDR and cell death in acquired radioresistant cells. (A) Double immunostaining with p-DNA-PK and PCNA in HepG2 cells is shown in the upper panel. Double immunostaining with p-ATM and PCNA in HeLa cells is shown in the lower panel. (B) Colony survival of HeLa cells treated with 10 μM NU7026 or 1 μM KU55933. Asterisk indicates significant sensitivity to drugs by 31FR-31NR cells compared with 0FR cells (C) Percentage of annexin V-positive HeLa cells. The cells were treated with either NU7026 or KU55933. Asterisk indicates a significant difference in the frequency of apoptotic FR cells compared with 0FR cells.