Figures & data
Figure 1. Identification of PRDX1 as a Pin1 binding protein. (A) Lysates of HEK293 cells were incubated with mock Ni resin (Lane 1) or Pin1-His-bound Ni resin (Lane 2). Eluted Pin1-His protein complex was analyzed by SDS-PAGE and visualized by silver staining. The identities of Pin1 binding proteins were determined by mass spectrometry. (B) 293T cells were co-transfected with FLAG-Pin1 and HA-PRDX1-WT expression plasmids. Lysates of the transfected 293T cells were prepared two days post-transfection for IP analysis. IP was performed using anti-FLAG or anti-HA antibodies followed by western blotting. IP with a normal IgG was used as a control. (C) 293T cells were transiently co-transfected with FLAG-Pin1 and HA-PRDX1-T90A mutant plasmids. Forward and reverse IPs were performed using the lysates of the transfected cells, followed by Western analysis. (D) Cell lysates prepared from 293T cells were analyzed by IP using an anti-Pin1 antibody, followed by western blotting with an anti-PRDX1 antibody.
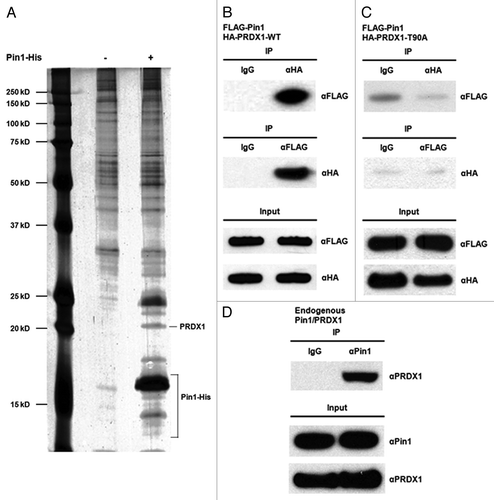
Figure 2. Sub-cellular co-localization of PRDX1 and Pin1. HeLa cells were co-transfected with HA-PRDX1/FLAG-Pin1 plasmids and immunostained with anti-HA (green) and anti-FLAG (red) antibodies. Nuclei were visualized by DAPI.
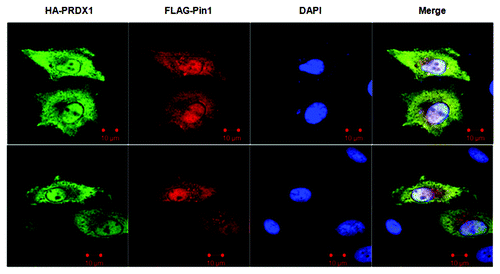
Figure 3. Regulation of PRDX1 peroxidase activity by Pin1. (A) The amounts of H2O2 release in the cultured media of Pin1 WT and Pin1 KO MEFs. Measurement of H2O2 was described in Materials and Methods. Error bars indicate standard deviations. (B) Peroxidase activity of PRDXs in Pin1 WT and Pin1 KO MEFs. (C) Pin1 WT and Pin1 KO MEFs were transfected with FLAG or FLAG-Pin1 plasmids as indicated. PRDX peroxidase activities of the transfected cells were measured as described in Materials and Methods. Expression of endogenous Pin1 and FLAG-Pin1 was examined by western blotting.
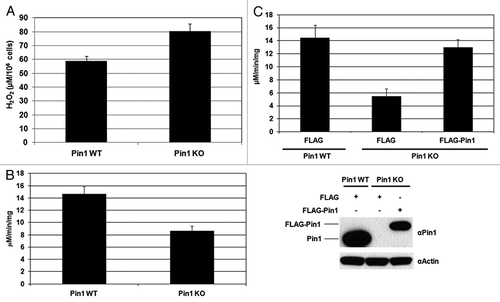
Figure 4. Pin1 interacts with PRDX2–4. (A) Lysates of the 293T cells co-transfected with FLAG-Pin1 and an indicated wild-type PRDX expression plasmids (i.e., HA-PRDX2, HA-PRDX3and HA-PRDX4) were used for IP analysis. IP with an anti-HA antibody was performed followed by western blotting using an anti-FLAG antibody. IP with a normal IgG was used as a negative control. (B) 293T cells were co-transfected with FLAG-Pin1 and an indicated mutant PRDX expression plasmid (i.e., HA-PRDX2-T89A, HA-PRDX3-T146A and HA-PRDX4-T162A). Lysates prepared from the transfected cells were analyzed by IP, followed by western blotting.
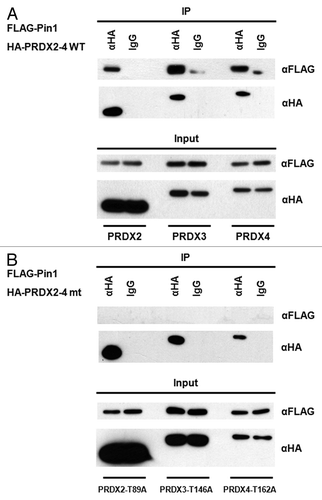
Figure 5. Pin1 facilitates the dephosphorylation of PRDX1 by PP2A phosphatase. (A) Cell lysates prepared from Pin1 WT and Pin1 KO MEFs were analyzed by IP using an anti-phospho-threonine-proline (αPhospho-TP) antibody. The presence of PRDX1 in the immunoprecipitated complexes was determined by western blotting with an anti-PRDX1 antibody. (B) Pin1 KO MEFs were transfected with an empty or a FLAG-Pin1 plasmid. Phosphorylation of PRDX1 at Thr90 was examined by IP with the αPhospho-TP antibody. (C) PP2A assay was performed by incubation of PP2A, purified Pin1-His proteins (10, 20 and 40 µg), and a synthetic PRDX1 peptide containing phospho-Thr90-Pro91 residues for 10 min. (D) A similar set of PP2A assay was performed with PP2A, 10 µg Pin1 protein and a synthetic phospho-PRDX1 peptide. The reaction without Pin1 protein was used as a control. The activities of PP2A were measured at different time points (0, 15 and 30 min).
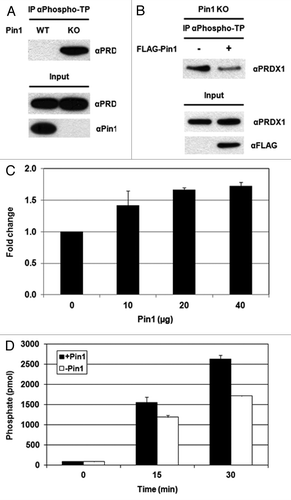
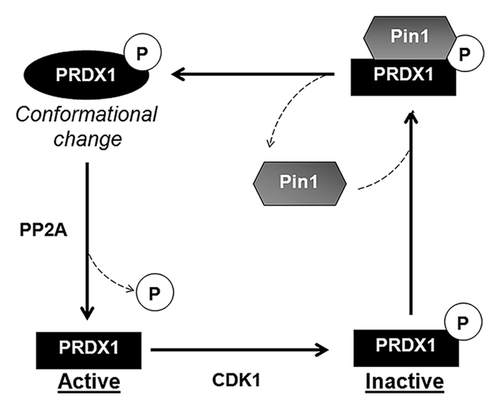
Figure 6. Proposed model for the regulation of PRDX1 activity by Pin1. CDK1 phosphorylates PRDX1 at Thr90 and inactivates PRDX1 peroxidase activity. Pin1 binds to the phosphorylated PRDX1 through the phospho-Thr90-Pro91 motif and induces isomerization of PRDX1. The protein conformational change of PRDX1 caused by Pin1, stimulates the dephosphorylation of PRDX1 at Thr90 by PP2A phosphatase, leading to the re-activation of PRDX1 activity.