Figures & data
Figure 1. Ezh2, Suz12, and Eed recruit to laser-induced DNA damage. U2OS cells expressing GFP-Ezh2, GFP-Suz12, or GFP-Eed were laser micro-irradiated and monitored using time-lapse microscopy. Representative images at 0, 2, and 100 s are shown after laser micro-irradiation. Accumulation of GFP-Ezh2, GFP-Suz12, and GFP-Eed on the laser damage tracks over time were quantified and plotted (n = 20). Increased fluorescence on the damage tracks is plotted over time. Ezh2 does not recruit to UV damage. (B) U2OS cells were grown on a coverslip for 24 h prior to the experiment. The coverslip was then placed below a polycarbonate membrane and exposed to UV radiation (120 J/m2). Cells were then left to recover for 20 min and then fixed with 4% PFA. Cells were immuno-stained with Ezh2 and γ-H2AX antibodies. (C) U2OS cells were transfected with GFP-Ezh2 24 h before the experiment. Cells were either treated with UV (120 J/m2), MNNG (100μM) or left untreated and fluorescent recovery after photobleaching was then performed to determine the kinetics of GFP-Ezh2. Relative intensity of the bleached region was plotted over time. (D) Ezh2 recruitment is not dependent on H2AX, ATM, or DNA PK. GFP-Ezh2 was transfected in H2AX KO, EBS, or MO59J cells 24 h before the experiment. Cells were then laser micro-irradiated and imaged over time. Representative images at three different time points are shown and quantifications (an average of 12–15 cells was plotted) of the micro-irradiated regions were shown on the right.
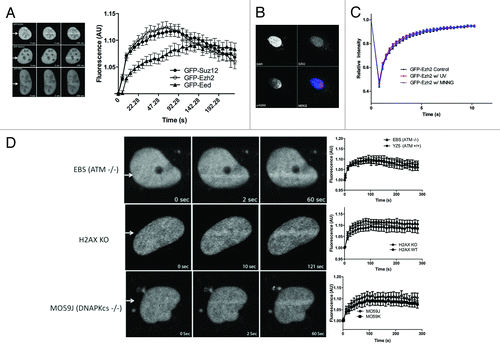
Figure 2. Ezh2 recruitment to laser induced DNA damage is PARP dependent. U2OS cells expressing (A) GFP-Ezh2, (B) GFP-Eed, or (C) GFP-Suz12 were treated with PARP inhibitor AG14361 (5 μM) or DMSO (control) for 1 h prior to damage induction. Cells were then laser micro-irradiated and imaged over time. Quantifications (an average of 12–15 cells was plotted) of the GFP signal at the micro-irradiated regions were plotted and shown as indicated. Error bars represent SEM.
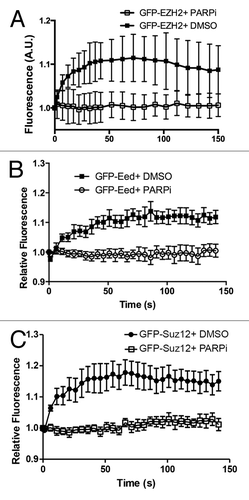
Figure 3. H3K27 is not methylated upon laser induced DNA damage. (A) U2OS cells were laser micro-irradiated and then fixed with 4% paraformaldehyde 5 min after laser induced DNA damage. Cells were then co-immunostained with H3K27me3 and γ-H2AX antibodies. (B) U2OS cells were exposed to 2 Gy and then left to recover for 30 min. Cells were then fixed with 4% PFA and co-immunostained with H3K27me3 and γ-H2AX antibodies. (C) U2OS cells were either not treated or treated with 8 Gy and left to recover for 30 min before harvesting. Nuclei were extracted, sonicated, and run on an 18% polyacrilamide gel. The western blots were stained with H3K27me3 and γ- H2AX to show the induction upon ionizing radiation.
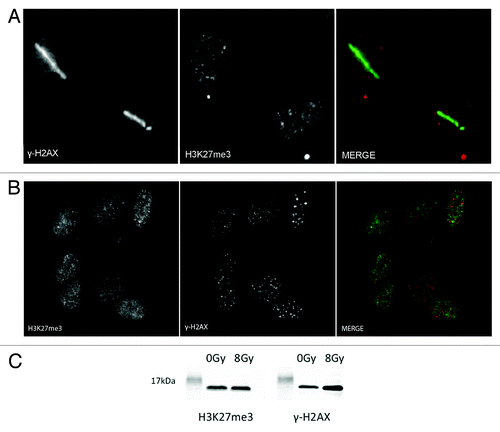
Figure 4. Ezh2 does not accumulate at IRIF. U2OS cells expressing GFP-Ezh2 were exposed to 2 Gy and left to recovery for 30 min. Cells were then fixed in 4% paraformaldehyde and co-immunostained with γ-H2AX and Ezh2 antibodies.
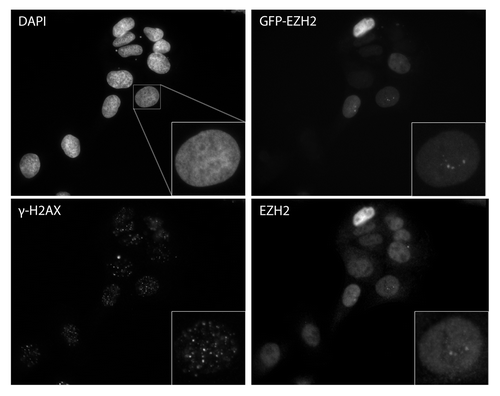
Figure 5. Ezh2 accumulates at Fok-1 induced DSBs. U2OS cells stably expressing the reporter locus were transfected with wild-type Fok-1 (FOK1 WT) or Fok1 catalytically inactive mutant (FOK1 mut) endonuclease. Cells were then fixed with 1% paraformaldehyde 18m h after transfection and harvested for chromatin immunoprecipitation (Ch-IP). Ch-IP was performed with Ezh2, γ-H2AX, and IgG antibodies. Quantitative PCR using 5 representative primers was done, and the Fok-1 DSB induced enrichment for each primer and antibody is plotted.
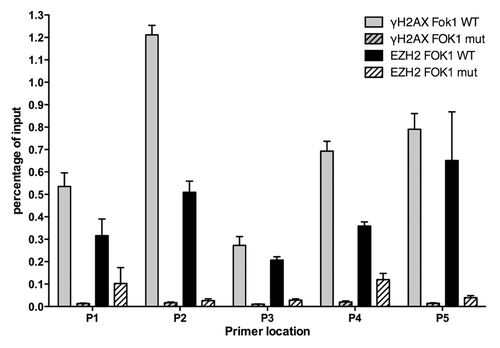
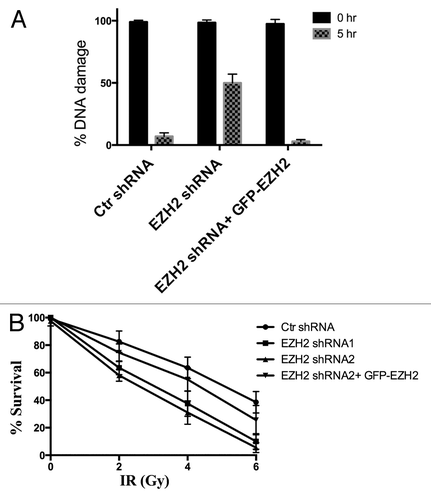
Figure 6. Ezh2 knockdown decreases DSB repair efficiency and increases cellular sensitivity to IR. (A) U2OS cells stably expressing Ezh2-shRNA, and U2OS cells stably expressing Ezh2-shRNA reconstituted with shRNA-resistant GFP-Ezh2 construct were exposed to 40 Gy ionizing radiation. Cells were harvested immediately to determine total damage and after 5 hrs to determine the DNA damage remaining. Total DNA damage immediately after IR is referred to as 100% DNA damage. (B) U2OS cells transfected with control shRNA, two different Ezh2- shRNAs, or Ezh2-shRNA reconstituted with shRNA -esistant GFP-Ezh2 construct were plated at low density. Cells were then exposed to 0, 2, 4, or 6 Gy and left to recover for 2 wk. The cells were then fixed and stained and colonies were counted. Each colony represents a surviving cell, and the percent survival is plotted for each dose of IR.