Figures & data
Figure 1. Structure and expression of HOPS. (A) Linear gene structure of the mouse Hops. In mouse, Hops is sited in chromosome 4 between genes AGAP 3 and FASTK. The relative sizes and position of exons are shown in blue, and coding sequences are in red bars. (B) Expression of Hops mRNA in different human tissues. Human multiple tissue expression array was hybridized with cDNAs specific for Hops. The arbitrary units values in the figures are defined by a ratio where the highest Hops mRNA expression was taken as 100% (mammary gland Hops mRNA) and the gene expression of Hops mRNA of other tissues. (C) Schematic representation of HOPS mouse protein. HOPS shows the presence of 2 methionines spaced by 55 amino acids. Upstream and downstream of the second methionine the position of the epitopes of the 2 specific antibodies raised for HOPS, PG105 and PG124, are indicated. Relevant functional domains are highlighted together with the amino acidic position. (D) Immunofluorescence analysis. Cells, transfected with a plasmid that expresses HOPS were fixed 24 h after transfection and decorated with antibodies anti-HOPS. Top: cells were stained with the HOPS PG124 antibody. Bottom: cells were stained with the HOPS PG105 antibody. Bars indicate 10μm. (E) Western blot analysis to detect HOPS isoforms. Protein lysates of cells transfected with Hops cDNA were probed using HOPS PG124 and HOPS PG105 antibodies. The immunoblotting with HOPS PG124 antibody shows the presence of 3 specific isoforms of HOPS: lHOPS, iHOPS, and sHOPS. The detection performed with HOPS PG105 revealed only the isoforms IHOPS and iHOPS. The control was performed with the transfection of the empty plasmid. Tubulin antibody was used as loading control. (F) Proteins extracted from cells transfected with cDNAs encoding sHOPS, lHOPS and HOPS M55V were analyzed with HOPS PG124 and HOPS PG105 antibodies. The HOPS PG124 antibody reveals sHOPS, the 3 HOPS isoforms in cell transfected with lHOPS, and recognize in cell transfected with the mutant HOPS M55V, IHOPS and iHOPS isoforms. The HOPS PG105 antibody is not able to recognize the isoform sHOPS. Tubulin antibody was used as loading control. (G) HOPS expression in protein extracts of different mouse tissues. Western blot analysis was performed with the HOPS PG124 antibody that recognizes all 3 isoforms of HOPS. Tubulin antibody was used as loading control.
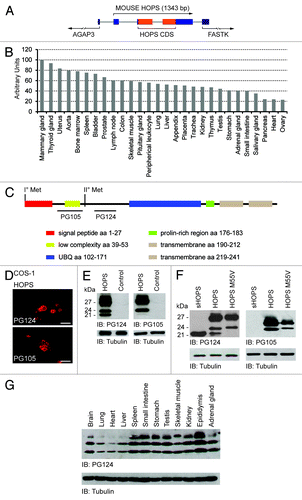
Figure 2. Study of iHOPS production. (A) Analysis of signal peptide of HOPS. Left: analysis of the cleavage sites of HOPS. Representation of the SPP site prediction of HOPS (top) and of the mutant HOPS Δ2–30 (bottom) by Signal 4.1 program. Right: western blot analysis of proteins extracted from cells transfected with HOPS, HOPS Δ2–30 and sHOPS cDNAs. The control has been performed with the transfection of the empty plasmid. For the detection - HOPS PG124 and HOPS PG105 antibodies have been used. Tubulin antibody was used as loading control. (B) Analysis of the GFP-HOPS and HOPS-GFP mutants. Top: schematic representation of the structure of the GFP-HOPS and HOPS-GFP mutants. Bottom: Left, localization of mutant proteins in COS-1 cells transfected with GFP-HOPS and HOPS-GFP. Cell transfected with plasmids pEGFP C1 and pEGFP N1 were used as control. All transfected cells were stained with the HOPS PG105 antibody. Bars indicate 10 μm. Middle, western blot analysis of protein lysates of cells transfected with pEGFP C1 GFP-HOPS, pEGFP N1, HOPS-GFP, and HOPS using HOPS PG124, and GFP antibodies. Control: empty plasmid transfection. Tubulin antibody was used as loading control. Right, blot analysis of proteins extracted from cells transfected with HOPS and myc-HOPS cDNA using HOPS PG124 antibody. Transfection of myc-HOPS is shown at 24 and 48 h. Tubulin antibody was used as loading control. (C) Analysis of the mutant HOPS-ΔUBL. Top: schematic representation of the HOPS-ΔUBL mutant. Bottom: Left and middle, HOPS immunofluorescence analysis in COS-1 cells transfected with HOPS-ΔUBL cDNA and HOPS cDNA as control (control). Antibodies against GFP and PG124 have been used for the detection. Bars indicate 10 μm. Right, western blot analysis of proteins extracted from cells transfected with the HOPS-ΔUBL and pEGFP C1 cDNA using anti-GFP antibody. The transfection of the empty plasmid has been performed as control. Tubulin antibody was used as loading control.
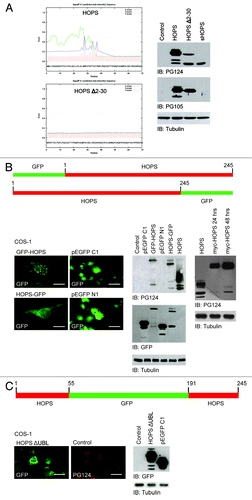
Figure 3. Structure and expression of HOPS isoforms. (A) Analysis of the GFP-sHOPS and sHOPS-GFP mutants. Top: schematic representation of the GFP-sHOPS and sHOPS-GFP mutants. Bottom: Left, immunofluorescence analysis of COS-1 cells transfected with GFP-sHOPS and sHOPS-GFP cDNA. For the detection GFP antibody has been used and the nuclei were stained with DAPI. The localization of GFP-sHOPS and sHOPS-GFP is shown in the merged image. Bars indicate 10 μm. Right, western blot analysis of proteins extracted from cells transfected with GFP-sHOPS, pEGFP C1, sHOPS-GFP, and pEGFP N1 using the GFP antibody. The control was performed with the transfection of the empty plasmid (control). Tubulin antibody was used as loading control. (B) HOPS in membrane of hepatoma cells. Western blot analysis of proteins of membrane extracted from whole cells and nuclei of H-35 cells. For the detection HOPS PG124 antibody has been used. Lamin B1 receptor (LBR) was used as control of the extraction of membranes. (C) HOPS in a cytosolic complex of hepatoma cells. Western blot analysis on cytosolic complex extracts resulting from H-35 cells fractionated by a continuous sucrose gradient.
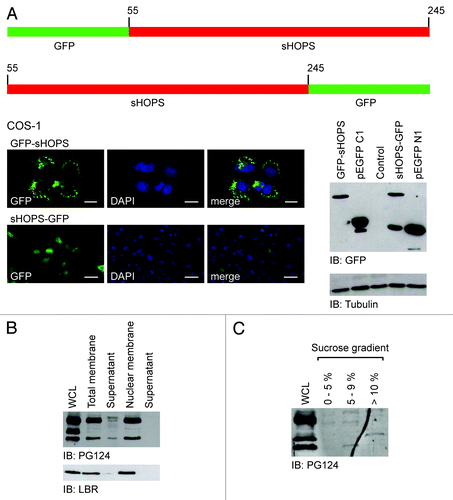
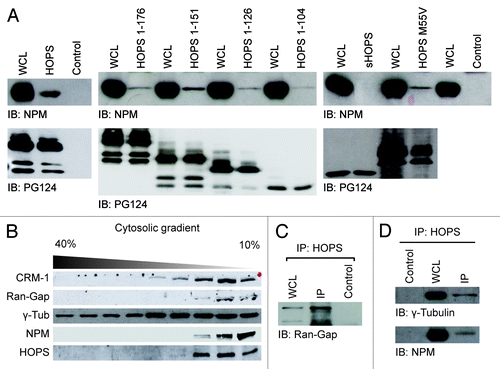
Figure 4. HOPS and NPM interaction. (A) Analysis of the binding between HOPS and NPM. Extracts of COS-1 cells transfected with cDNA of HOPS and HOPS mutants: HOPS 1–176, HOPS 1–151, HOPS 1–126, HOPS 1–104, sHOPS, and HOPSM55V, were immunoprecipitated with HOPS PG124 antibody that recognizes all HOPS isoforms. HA-irrelevant antibody was used as negative control (control). Whole-cell lysate (WCL) and resulting immunocomplexes were resolved by SDS-PAGE, transferred to membrane and probed with anti-NPM or HOPS PG124 antibodies. (B) Co-sedimentation of iHOPS with CRM-1, Ran-Gap, γ-Tubulin, NPM in the cytosol. Western blot analysis of cytosolic extracts from hepatoma cells fractionated by a 40–10% continuous sucrose gradient using CRM-1, Ran-Gap, γ-Tubulin, NPM, and HOPS PG124 antibodies. (C) Immunoprecipitation of cytosolic extracts of the fractions to 10% of the sucrose gradient. Western blot analysis of immunoprecipitation carried with HOPS PG124 antibody or HA irrelevant antibody as a negative control (Control) and resolved with Ran–Gap antibody. (D) Western blot analysis of immunoprecipitation of cytosolic extracts of the fractions to 10% of the sucrose gradient performed with HOPS antibody or anti-HA irrelevant antibody as a negative control (control) and resolved with anti γ-tubulin (top) or anti NPM antibodies (bottom).