Figures & data
Figure 1. Rsf-1 is recruited at DNA double-strand break site(s). (A and B) U2OS cells were immunostained with anti-Rsf-1 and anti-γH2AX at 2 h after treatment of phleomycin (+Phleo) (50 μg/ml) or untreatment of phleomycin (-Phleo). Scale bar, 10 μm. The damage foci were analyzed in 2-dimentional (2D) and 3-dimentional (3D) image with Workstation (Nikon). (C) U2OS and RPE1 cells were pre-sensitized with BrdU (10 μM) for 30 h, followed by laser micro-irradiation. Both cell lines were immunostained with anti-Rsf-1 and anti-γH2AX at 10 min after micro-irradiation. (D) Diagram of the recruitment of GFP-fused proteins and mCherry-LacI-FokI (wild-type and D450A mutant) on chromosome 1p3.6, stably integrated with LacO repeats, and co-localization of GFP-fused proteins and FokI wild-type (WT) or FokI mutant (D450A) in live cells. H2AX-GFP and Mre11-YFP used as controls. (E) Enrichment of Rsf-1, SNF2h, and γH2AX on site-specific DSB on chromosome 3. DSB was induced by transfection with K230-ZFN in U2OS cells, and fold enrichment by ChIP was divided by input.
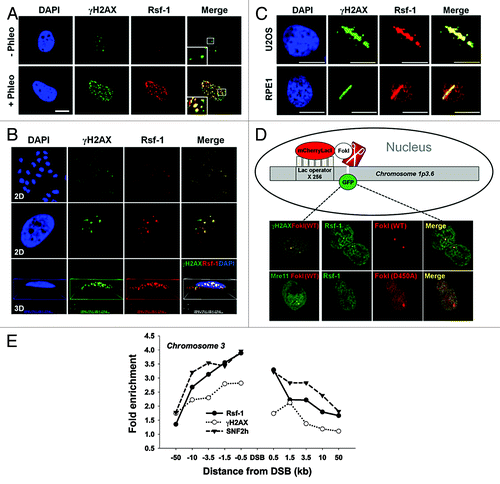
Figure 2. The kinetics of accumulation of Rsf-1, SNF2h, and γH2AX at DSBs. (A) U2OS cells were transfected with Rsf-1-GFP, SNF2h-GFP, or H2AX-GFP, and treated with BrdU (10 μM) for 30 h, followed by laser micro-irradiation. Scale bar, 10 μm. (B) Quantitative analysis of the fluorescence intensity in result (A). The fluorescence intensity at micro-irradiated sites was normalized by the intensity around the background. The normalized fluorescence units were plotted against time. The plotted values are mean ± SEM of more than 10 individual cells.
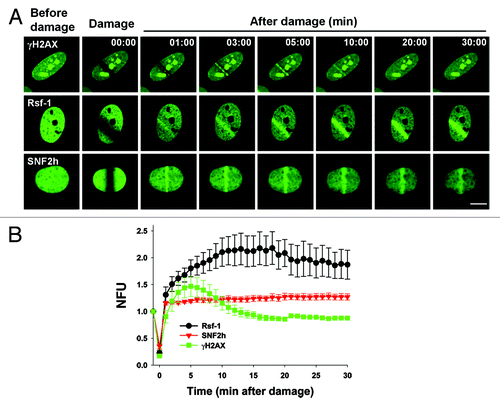
Figure 3. The accumulation of Rsf-1 at DSBs is dependent on ATM activity. (A) U2OS cells were untreated (Ctrl) or treated with ATM inhibitor (KU55933) (20 μm) prior to phleomycin treatment (+Phleo) or untreatment (−Phleo) and immunostained with anti-pATM (pS1981) and anti-Rsf-1. (B) Quantitative analysis of result in (A). “Before” means untreatment of phleomycin; “after” means treatment of phleomycin. The average number (mean ± S.E.M.) of foci per cell was counted from over 100 cells. ***P < 0.005 by Student t test. (C) Human fibroblast wild-type (WT) or ATM knockout (KO) cell was untreated (−Phleo), or treated with phleomycin (+Phleo) for 2 h and immunostained with anti-γH2AX and anti-Rsf-1. Scale bar, 10μm. (D) Quantitative analysis of result in (C). The percentage of foci-positive cells (>10 foci per cell) was counted. **P < 0.01, ***P < 0.005 by Student t test. (E) U2OS cells were transfected with EGFP, shATM, or shATR and pre-sensitized with BrdU for 30 h, followed by laser micro-irradiation. Cells were immunostained with anti-Rsf-1 and anti-pATM at 1 h after micro-irradiation. Scale bar, 10 μm. (F) The level of pATM (pS1981) and pATR (pS428) was analyzed by western blot.
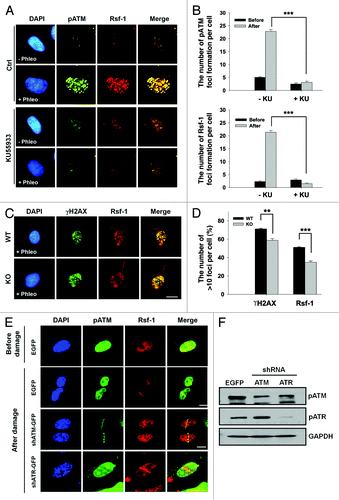
Figure 4. The putative pSQ motifs of Rsf-1 by ATM are required for its accumulation at DSBs. (A) U2OS cells were irradiated at 10 Gy and harvested at 1 h after laser micro-irradiation. Whole-cell extracts were lysed and immunoprecipitated with Rsf-1 antibody. The immunoprecipitated was detected with pSQ/TQ antibody. γH2AX antibody used as a DNA damage control. (B) Rsf-1-GFP (WT) or Rsf-1 mutant (3SA) was transfected in U2OS cells and pre-sensitized with BrdU for 30 h, followed by laser micro-irradiation. ATM inhibitor KU55933 was pre-treated for 12 h prior to micro-irradiation. Scale by bar, 10 μm. (C) Quantitative analysis of fluorescence intensity at DSBs. The accumulated fluorescence intensity at micro-irradiated sites was normalized the background around the sites. The values (mean ± S.E.M.) were calculated and plotted against time from at least 10 individual cells.
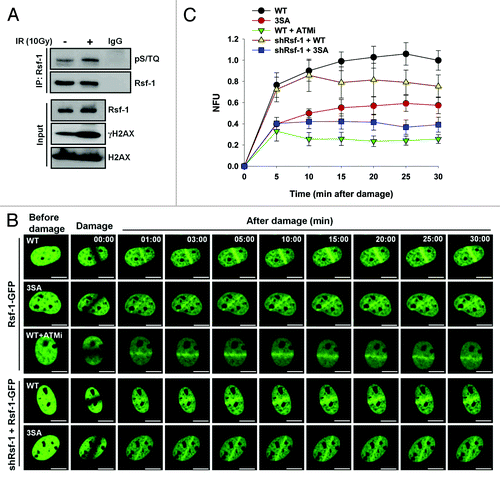
Figure 5. Depletion of Rsf-1 attenuated DNA damage checkpoints. (A) Stably Rsf-1-depleted (shRsf-1) or control (shCtrl) cells (HeLa) were treated with phleomycin (50 μg/ml) and harvested at each indicated time points. Whole-cell extracts were directly lysed with sample buffer. The activation of DNA damage checkpoints was examined by western blot with the indicated antibodies. U, untreated. (B) FACS profiles of stably Rsf-1-depleted (shRsf-1) or control (shCtrl) cells during continuous DNA damage with phleomycin. (C) Stably Rsf-1-knockdown or control U2OS cells were immunostained with anti-Rsf-1 and anti-γH2AX, or anti-Rsf-1 and anti-pATM at 1 h after laser micro-irradiation. Scale bar, 10 μm. (D) H2AX wild-type (WT) or H2AX mutant (S139A) was transfected into U2OS cells. Cells were immunostained with anti-Rsf-1 at 10 min after micro-irradiation. (E) Stably Rsf-1-depleted cells (U2OS) were treated with phleomycin for 2 h and immunostained with anti-γH2AX, anti-MDC1, and anti-53BP1. The average number (mean ± S.E.M.) of foci per cell was counted from over 100 cells. **P < 0.01, ***P < 0.005 by Student t test. (F) Stably Rsf-1-knock-downed U2OS cells were transfected with EGFP, Rsf-1 WT, and Rsf-1 3SA mutant and immunostained with anti-γH2AX, anti-MDC1, and anti-53BP1. The number of foci formation per cell was calculated. ***P < 0.005 by Student t test. N.S., not significant.
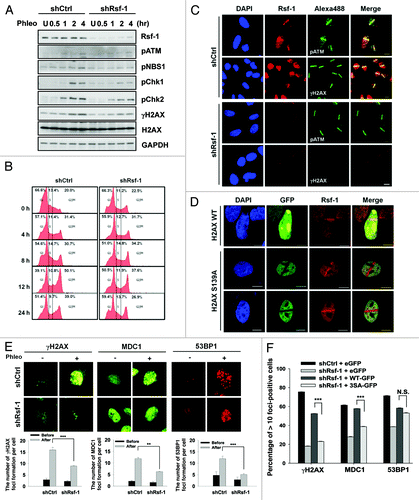
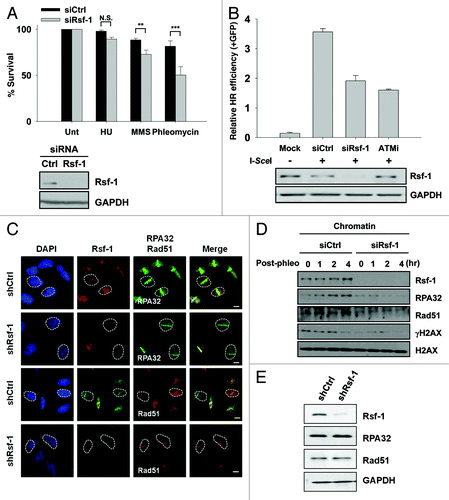
Figure 6. Rsf-1 facilitates homologous recombination repair. (A) The number of U2OS cells transfected with siCtrl and siRsf-1 was seeded and treated with different kinds of drugs; HU (100 mM), MMS (0.0002%), and phleomycin (50 μg/ml). The relative percentage of cell survival in control (siCtrl) and Rsf-1-depleted (siRsf-1) cells was calculated. N.S., not significant. **P < 0.01, ***P < 0.005 by Student t test. Expression level of Rsf-1 in whole-cell lysates was confirmed by western blot. (B) DR-GFP cells were transfected with control siRNA (siCtrl) or Rsf-1 siRNA (siRsf-1). At 48 h after siRNA transfection, I-SceI was transfected to induce DSB. ATM inhibitor (KU55933) was treated before transfection of I-SceI. GFP-positive cells were counted using FACS at another 72 h. (C) U2OS Rsf-1-depleted cells were pre-sensitized with BrdU for 30 h, followed by laser micro-irradiation. These cells were immunostained with anti-Rsf-1 and anti-RPA32 or anti-Rsf-1 and anti-Rad51, and imaged using confocal microscope. (D) RPE1 cells were transfected with Rsf-1 siRNA or control siRNA for 72 h and treated with phleomycin (10 μg/ml) for 1 h. The chromatin fractions were isolated at each time points released after treatment with phleomycin. The level of chromatin-bound proteins was analyzed by western blots. (E) The total level of Rsf-1, RPA32, and Rad51 in stably Rsf-1-depleted and control cells (U2OS).