Figures & data
Figure 1. Expression of map9 in early zebrafish embryos. (A) Relative quantification of map9 mRNA by qPCR in 0 hpf (unfertilized eggs) to 24 hpf embryos. Data are the mean ± SD from 3 independent experiments, with n = 25–35 embryos per time point (*P < 0.005 by Student t test). (Band C) Subcellular localization of Map9 during mitosis in 24 hpf embryos following expression of map9-YFP. Two hundred pg of map9-YFP RNA was injected at the 1-cell stage, embryos fixed at 24 hpf, and stained with an anti-α-tubulin antibody (red) and Hoechst 33258. Confocal microscopy images show that Map9-YFP (green) co-localizes with α-tubulin on the microtubules of the mitotic spindle (B). In interphase cells (C), Map9-YFP co-localizes with γ-tubulin (red) at centrosomes. (Scale bars, 10 μm). (D) Loss of the fiber-like distribution of Map9 lacking its C-terminal MAP domain in mitotic cells. Map9-YFP or map9ΔCter-YFP RNA (200 pg) was injected in 1-cell stage embryos. Embryos were fixed at 24 hpf and stained with anti-α-tubulin, anti-GFP antibodies, and Hoechst 33258. Confocal microscopy images show that Map9-YFP but not Map9ΔCter-YFP co-localizes with α-tubulin on the microtubules of the mitotic spindle. (Scale bars, 10 μm).
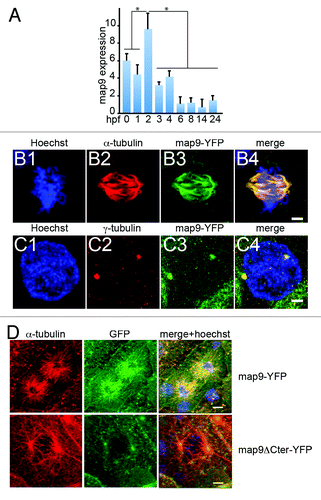
Figure 2. MO-mediated depletion of map9 leads to early defects and/or embryo death and growth failure. (A) Injection of splice-blocking MOex5 leads to the production of a mis-spliced map9 mRNA. RT-PCR was performed using map9 specific primers and RNA from embryos injected at the 1-cell stage with MOex5 (5 and 8 hpf), translation-blocking MO-ATG (8 hpf), or the control MOmis (8 hpf). PCR products were separated on 0.8% agarose gels. MOex5 PCR products show 2 bands that differ by ~300 bp in size. (B) Top: subcloning and sequencing of these 2 fragments revealed that the upper band corresponds to wild-type (wt) map 9, whereas the lower band corresponds to an mRNA in which exon 5 and 6 are deleted. mRNA from MO-ATG and MOmis-injected embryos show only wt map9. The position of MOex5 is indicated by an arrow on the acceptor splice junction of exon 5. Exons 5 and 6 are shaded in gray, and black boxes represent untranslated regions. Bottom: measure by qPCR of the expression of the 2 map9 mRNA forms in 8 hpf MOex5 and MO-ATG morphants. In MOex5 morphants the expression of map9 is ~5 times lower than in MO-ATG morphants, and the misspliced forms represents 11% of map9 mRNA vs. background level < 1% in MO-ATG morphants (inset). (C–F) One-cell stage zebrafish embryos were injected with 1 pmol of anti-map9 MOex5 or control MOmis and imaged at mid-epiboly. Growth and epiboly in MOex5 morphants are arrested very early. (G–L) Embryos at 24 and 48 hpf after injection of 0.25 pmol of the 2 MOs. Map9 morphants show complex malformations, including underdeveloped nervous system, absence of the eyes, abnormal yolk, notochord (I and J, arrow in the insets), somites and tail (J, arrowheads), and pericardial edema (L, arrowhead). In (I and J), the insets are enlargements of the dotted boxes.
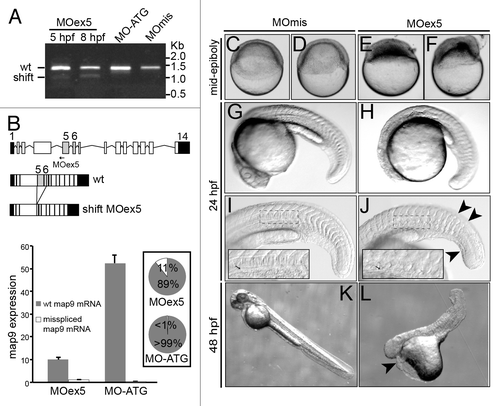
Figure 3.Map9-YFP RNA can partially rescue the phenotypes of MOex5 morphants. (A) Embryos were injected at the 1-cell stage with 1 pmol MOex5, 200 pg map9-YFP RNA or map9ΔCter-YFP RNA, or co-injected with MOex5 and either RNA. Map9-YFP- and map9ΔCter-YFP-injected embryos developed normally up to mid-epiboly and later stages (green). Forty % of MOex5 morphants showed defects at mid-epiboly (red), 20% were blocked at the sphere stage (blue), and all of them died at 9–10 hpf. Following co-injection of MOex5 and map9-YFP RNA, about 80% of embryos developed normally up to mid-epiboly but died before 24 hpf, whereas co-injection with map9ΔCter-YFP did not rescue the morphant phenotype (n = 64 for map9ΔCter-YFP-injected embryos; n = 54 for embryos co-injected with MOex5 and map9ΔCter-YFP RNA). (B) Representative images of embryos injected with map9-YFP RNA (at mid-epiboly, n = 102), with MOex5 (showing developmental delay and defects before mid-epiboly, n = 85), or with both MOex5 and map9-YFP RNA (showing partial rescue of the phenotype at mid-epiboly, n = 153). Scale bars, 200 μm.
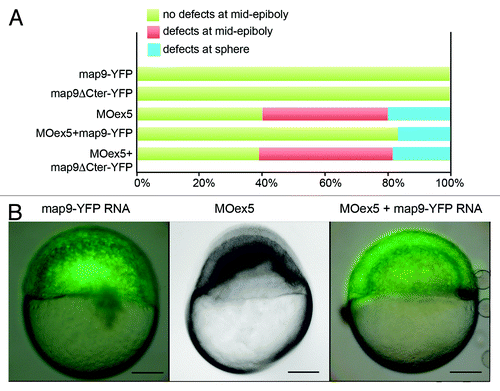
Figure 4. Morpholino-mediated depletion of map9 leads to increased apoptosis. (A–D) Zebrafish embryos were injected, or not (control), at the 1-cell stage with 1 pmol of MOex5 for observation at 8 hpf, or with 0.25 pmol MOex5 for observation at 24 hpf. TUNEL-positive cells (green) were counted in injected (B and D) and control embryos (A and C) (n = 10/assay). (E) Left panel, number of TUNEL-positive cells in the control and MOex5-injected embryos depicted in (A–D), n = 5 embryos per assay, total cell count ~1070 (*P < 0.005, **P < 0.02); right panel, tp53 inhibition does not rescue apoptosis induced by MOex5. Number of TUNEL-positive cells in MOex5- and MOex5 + MOtp53-injected embryos (n = 20 embryos per assay, total cell count ~564). (F) Map9 depletion does not induce tp53 expression. Embryos were injected at the 1-cell stage with 1 pmol of MOex5. RNA from 7 hpf map9 morphants and control embryos was used to quantify tp53 gene expression by RT-qPCR. Expression data were normalized to β-actin. Experiments were made in quadruplicate, n = 42 control embryos, and 50 MOex5 morphants at 7 hpf, and n = 39/44 embryos at 24 hpf. (G) Co-injection of MOex5 (1 pmol) and MOtp53 (0.5 pmol) does not rescue MOex5 effects. Embryo mortality was not rescued after tp53 depletion by morpholino. Embryo mortality after injection of a control MO (MOmis) was not significantly different compared with non-injected embryos. This experiment was performed in duplicate.
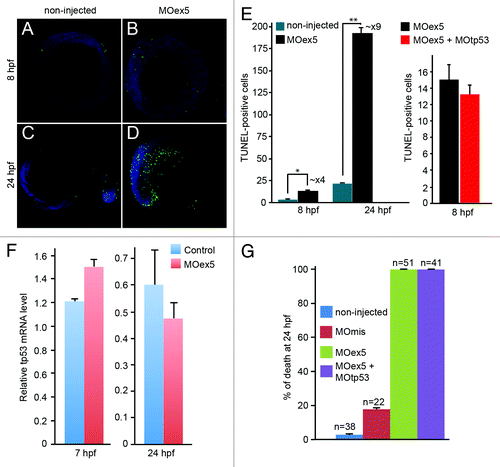
Figure 5. Morpholino-mediated depletion of map9 leads to a reduction in the number of mitotic cells and to mitotic defects. (AandB) Embryos were injected at the 1-cell stage with 1 pmol MOex5 or MOmis (control) and stained with the anti-phosphorylated histone H3 (pH3) antibody (red). DNA was labeled with Hoechst 33258 (blue). Scale bars, 200 μm. (C) pH3-positive mitotic cells were counted in injected embryos at 5 hpf (n = 10/group). In MOex5 morphants the number of mitotic cells was reduced by ~40% in comparison to controls. Similar results were obtained in 8 hpf embryos (not shown). (D–G) Embryos were injected at the 1-cell stage with 1 pmol MOmis (control) (D and E) or MOex5 (F and G) and mitotic cells were imaged in 8 hpf embryos by confocal microscopy (scale bars, 10 μm). DNA was labeled with Hoechst (green) and MTs of the mitotic spindles with an anti-α-tubulin antibody (red). (H) Percentage of cells at different phases of mitosis. Mitotic cells were scored in map9 morphants and control embryos (n = 10 embryos/assay).
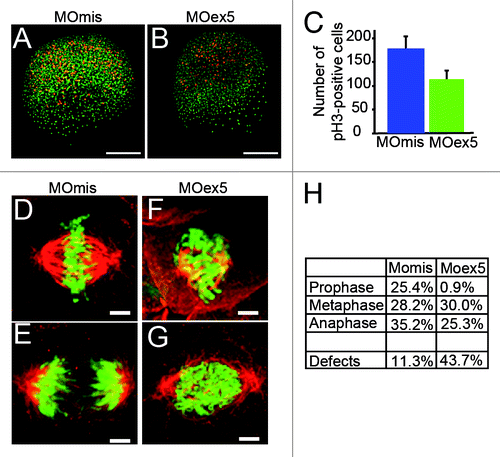
Figure 6. Microtubules in the YSL are disorganized in map9 morphants. (A) Schematic representation of the organization of the YSL and yolk cell MT array in early zebrafish embryos (adapted from ref. Citation43). The portion boxed in blue represents the region analyzed in (B– D). (B andC) Embryos were injected at the 1-cell stage with 1 pmol of control MOmis (B) or MOex5 (C) and imaged by confocal microscopy at ~4.3 hpf (beginning of epiboly). The MT spindles/array are dense and clearly identified in MOmis-injected embryos, whereas they are depleted in MOex5 morphants. (D) Map9 localizes on the MT network of the YSL. Map9-YFP, YFP, and map9 RNA (200 pg) were injected separately in 1-cell-stage embryos and imaged at 4.3 hpf. The MT network is labeled with an anti-α-tubulin antibody (red), and Map9-YFP is labeled with an anti-GFP antibody. Nuclei are stained with Hoechst 33258. (E) Map9-YFP RNA was injected in 1-cell stage embryos. Map9 colocalizes with α-tubulin on the mitotic spindles of YSL nuclei, (scale bars, 20 μm).
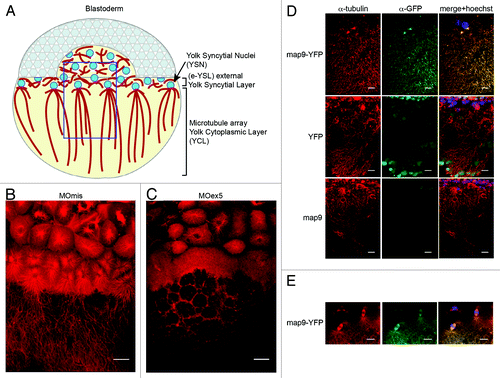
Figure 7. Inhibition of map9 expression in the YSL blocks epiboly. (A–L) At the beginning of YSL formation (23/4-3 hpf), 1 pmol of control MOmis or MOex5 and dextran-rhodamine (red) were co-injected in the YSL. Embryos were observed at 42/3 hpf (30% epiboly) and at ~8 hpf (80% epiboly). Scale bars, 200 μm.
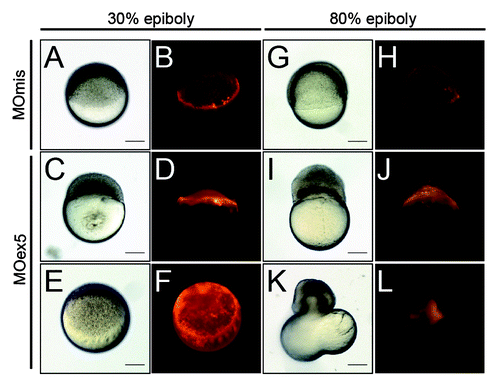
Figure 8. Depletion of map9 deregulates the expression of genes involved in different signaling pathways. Embryos were injected at the 1-cell stage with 1 pmol of MOex5 or MOmis (controls). RNA from 7 hpf map9 morphants and control embryos was used to quantify gene expression by RT-qPCR. Expression data were normalized to β-actin. Results are presented as the relative quantification of gene expression in MOex5 morphants and control embryos (C). For each gene, the morphant/control ratio is indicated below the graph, with the lowest value surrounded by a square. Inset, relative quantification of tuba8l, a maternal and miR-430 target gene whose expression during the early stages of development relies on miR-430 expression. Experiments were made in quadruplicate; n = 42 control embryos and 50 MOex5 morphants.
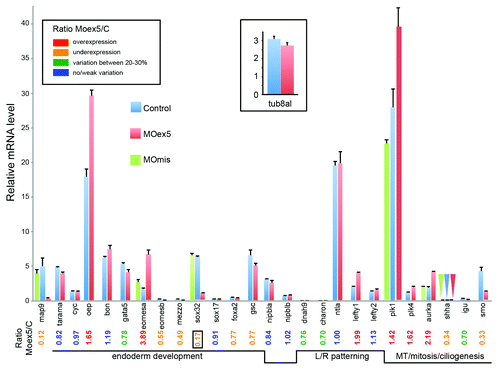
Figure 9. In situ hybridization of selected genes of the nodal pathway that are involved in endoderm development. Embryos injected at the 1-cell stage with 1 pmol of control MOmis or MOex5, were fixed at the indicated times that correspond in MOmis-injected control embryos to the following developmental stages: dome (41/3 hpf), germ-ring (52/3 hpf), and shield (6 hpf). In situ hybridization was performed using antisense oep (nodal co-receptor), sox32 (downstream of oep) and sox17 (activated by sox32 and required for endoderm specification) RNA probes. In map9 morphants, expression of sox32 and sox17 was restricted to a portion of the blastoderm margin at the shield stage (arrowheads, B8, C8).
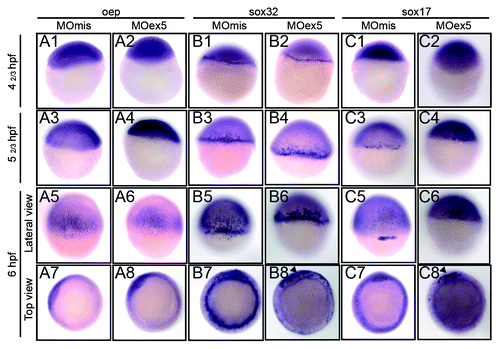
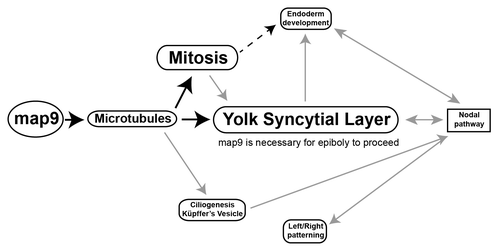
Figure 10. Tentative model for the role of Map9 in zebrafish development. Map9 is required for MT dynamics. MTs are key components of the mitotic spindle (mitosis) and cilia. MTs and mitosis are essential for the formation of the YSL and epiboly. YSL is required for endoderm development and nodal signaling and the cilia of the KV are responsible for propagating nodal signals involved in left/right asymmetry. As indicated on the schematic, most of these pathways are interdependent. Black arrows indicate the possible role of Map9 during zebrafish development suggested by this and previous studies,Citation9-Citation11,Citation19 and gray arrows summarize literature data.