Figures & data
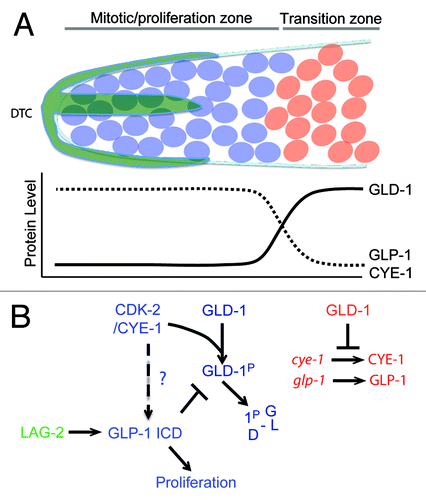
Figure 1. (A) Asymmetric segregation of cell fate determinants in asymmetric neuroblast cell division of Drosophila. Apical factors (such as Insecutable and Pins, and the evolutionary conserved Par complex consisting of Par-6, Bazooka, and Cdc42) shown in blue and basal factors (such as Miranda, Prospero, and Numb) shown in red are asymmetrically localized on the cortex along the apico–basal axis of neuroblast (left) and segregated asymmetrically to larger apical cell that retains neuroblast (NB) fate and smaller basal cell called ganglion mother cell (GMC). Mitotic spindle that orients along the apico–basal axis ensures asymmetric inheritance of the cortical cell fate determinants. Cdc2 activity is required to maintain the cortical asymmetry during cell division. (B) Asymmetric cell division of thoracic neuroblast 6-4 (NB6-4). NB6-4t divides asymmetrically to produce a neuroblast (blue) and gliablast (orange), whereas NB6-4a divides symmetrically giving rise to 2 glial cells. The neuroblast identity is maintained by high levels of Cyclin E (white stars), which is significantly reduced in NB6-4a.
Figure 2. (A) Alignment of amino acid sequences surrounding the T-loop region of CDK1 from worm, fly, and mouse, showing the positions of conserved amino acid sequence mutated in the hypomorphic alleles of cdk-1 (ne2257 [I173F] and ne236 [R176H]) isolated in genetic screens using C.elegans, boxed in red or yellow, respectively. Invariant amino acids are indicated with an asterisk. (B) Crystal structure of activated mouse CDK2/CyclinA complex. Amino acids corresponding to those affected in the hypomorphic alleles of cdk-1, Ile173 in the case of ne2257, and Arg176 in the case of ne236 are shown in red and yellow, respectively. The amino acids corresponding to those affected in 2 cyb-3 suppressor alleles of cdk-1(ne2257) are also shown in magenta (ne4276) and light blue (ne4277).
![Figure 2. (A) Alignment of amino acid sequences surrounding the T-loop region of CDK1 from worm, fly, and mouse, showing the positions of conserved amino acid sequence mutated in the hypomorphic alleles of cdk-1 (ne2257 [I173F] and ne236 [R176H]) isolated in genetic screens using C.elegans, boxed in red or yellow, respectively. Invariant amino acids are indicated with an asterisk. (B) Crystal structure of activated mouse CDK2/CyclinA complex. Amino acids corresponding to those affected in the hypomorphic alleles of cdk-1, Ile173 in the case of ne2257, and Arg176 in the case of ne236 are shown in red and yellow, respectively. The amino acids corresponding to those affected in 2 cyb-3 suppressor alleles of cdk-1(ne2257) are also shown in magenta (ne4276) and light blue (ne4277).](/cms/asset/0053db91-873e-400c-b6d1-17ad6f70d404/kccy_a_10928656_f0002.gif)
Figure 3. (A) Model for the role of CDK-1 in OMA-1 degradation during oocyte-to-embryo transition. CDK-1 functions together with conserved protein kinases, for example, MBK-2, to promote the timely destruction of zinc-finger protein OMA-1 during the first mitosis. Removal of OMA-1 allows the protein degradation machinery to downregulate the levels of various cell fate determinants for asymmetric inheritance and release of sequestered TAF-4, a component crucial for the assembly of the transcription factor-II D (TFIID) and the RNA polymerase II complex in zygotic gene expression. CDK-1 also functions during the progression through meiosis to phosphorylate and activate MBK-2, which is sequestered on the cortex by EGG-3/4/5, until anaphase-promoting complex degrades EGG-4/5 and releases MBK-2 to cytoplasm. Upon release from the cortex, MBK-2 phosphorylates OMA-1 and starts the sequential phosphorylation events; however, the destruction of OMA-1 is delayed until the first mitosis when CDK-1 is again activated. (B) Model for CDK-1 function in regulating asymmetric cell division of EMS cell. CDK-1 phosphorylation of WRM-1 together with modifications induced by Wnt signaling releases WRM-1, an inhibitor of spindle rotation, from the posterior cortex of EMS, thereby exposing a polarity cue (red solid line) that captures one of the astral microtubules (brown arrow) and rotates the mitotic spindle in alignment with the anterior–posterior axis of embryo. Wnt signal and Src signal reinforce the capturing and/or pulling of astral microtubule. Modified WRM-1 (for example by phosphorylation) released from the posterior cortex of EMS accumulates preferentially in the posterior nascent nucleus and drives the endodermal fate.
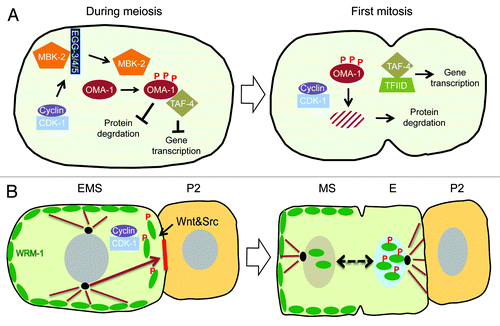
Figure 4. (A) Transition from mitosis into meiosis in C.elegans germline. The Distal Tip Cell (DTC in green) caps the distal end of the germline and induces mitotic proliferation (blue). Beyond the reach of the DTC Notch signaling, germ cells transition into the differentiation zone in a GLD-1 dependent manner, where they undergo meiosis (red). (B) Model for Cyclin E in maintenance of germ stem-cell fate in C. elegans. The boundary between the proliferative and transition zones is maintained by 2 mutual negative regulations: CDK-2/ CYE-1 and GLP-1 inhibit accumulation of GLD-1 in the mitotic proliferation zone, while GLD-1 represses the translation of cye-1 and glp-1 transcripts in the transition zone. It remains to be seen if CYE-1 can maintain proliferation zone by enhancing GLP-1 ICD induction in the similar way it does in vulval development.