Figures & data
Figure 1. Delayed mitotic entry upon treatment of synchronized Hela cells with variant C. difficile Toxin B. HeLa cells were synchronized using the thymidine double-block technique. HeLa cells were allowed to progress into G2 phase and were treated with variant Toxin B from the C. difficile serotype F strain 1470 (TcdBF) 8 h after release from the second thymidine block or left untreated. Cells were fixed every 2 h at the indicated time points post-release, and cell cycle progression was analyzed by FACS of propidium iodide-stained cells.
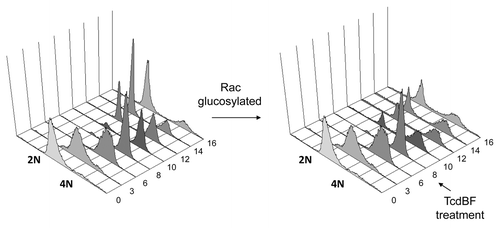
Figure 2. Rac1-dependent association of PAK2 with G2-phase centrosomes HeLa cells were synchronized by the thymidine double block. Cells were allowed to release from block and entered G2 phase 8 h after release from the second block and were left non-treated (A) or treated with ToxinB (30 ng/ml) for 2 h (B). Centrosomes were isolated using sucrose density ultracentrifugation and fractions were analyzed by western blotting for the indicated proteins. Centrosome-enriched fractions were indicated by γ-tubulin and referred to as isolated centrosomes. The γ-tubulin signal was strongest in fractions 3 and 4, corresponding to 60% (w/w) sucrose. Whole cell lysate (WCL) from G2-phase synchronized HeLa cells was applied as positive control, confirming the presence of the respective proteins within the cell lysate.
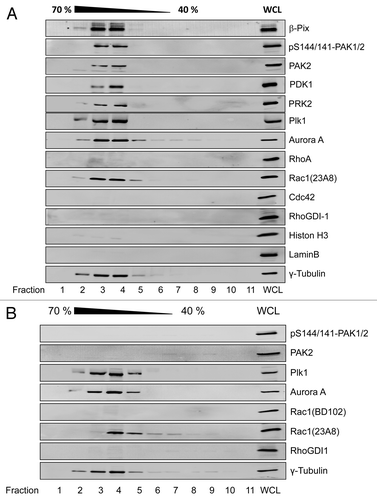
Figure 3. Cell cycle-dependent localization of Rac1 and pS144/141-PAK1/2 at the centrosomes. (A) Synchronized Hela cells were fixed with methanol and stained for Rac1 (green), γ-tubulin (red), and DNA (blue). Scale bar: 15 µm. (B) Synchronized Hela cells were fixed with methanol and stained for pS144/141-PAK1/2 (red), γ-tubulin (green), and DNA (blue). Scale bar: 15 µm. (C) Synchronized HeLa cells were treated with TcdB (30 ng/ml, ●), TcdBF (1 µg/ml, ▲), or buffer (■) 8 h after release from the block. Cells were stained for pS144/141-PAK1/2 (red), γ-tubulin (green), and DNA (blue). The number of cells with pS144/141-PAK1/2-positive centrosomes per total cells and of mitotic per total cells was quantified at the indicated time points by manual counting from 3 independent experiments with >100 cells in each population. Data are mean ± SD.
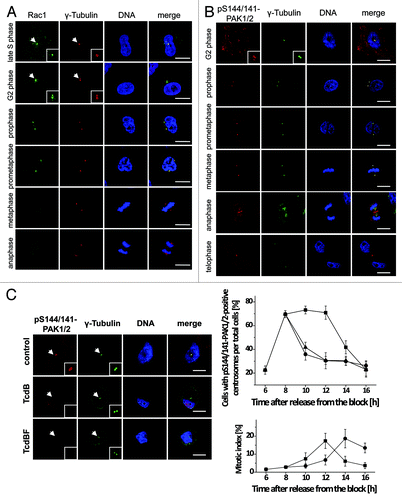
Figure 4. Delayed mitotic entry upon inhibition of Rac1 and PAK1/2. (A) Synchronized HeLa cells were treated with TcdBF (1000 ng/ml) in G2 phase 8 h after release from the second block. Cell lysates were collected at the indicated time points and were analyzed by western blot analysisusing cell cycle marker proteins. β-actin was used as loading control and Rac1 inactivation by glucosylation as well as PAK phosphorylation, indicative for PAK activity, was determined. (B) Activation of the CyclinB/Cdk1 complex was determined by an in vitro kinase assay. The CyclinB–Cdk1 complex was immunoprecipitated by anti-CyclinB1 antibody and incubated with [γ-32P] ATP in the presence of histone H1, as a Cdk1 substrate. Phosphorylation of histone H1 was determined by autoradiography. (C) Synchronized HeLa cells were transfected with siRNA targeting PAK2 (siPAK2a; siPAK2b), or with scrambled control siRNA (scr). Forty-eight h after transfection, mitotic entry was analyzed in synchronized HeLa cells by western blotting using cell cycle marker proteins. (D) Synchronized Rac1fl/fl MEFs were treated with TcdBF (30 ng/ml) in G2 phase 3 h after release from the second block. Cell lysates were collected at the indicated time points and were analyzed by western blot analysis using cell cycle marker proteins. β-actin was used as loading control and Rac1 inactivation by glucosylation as well as PAK phosphorylation, indicative for PAK activity, was determined. (E) Rac1fl/fl and Rac1−/− MEFs were synchronized by the thymidine double-block technique and analyzed for cell cycle progression by western blot analysis of cell cycle marker proteins.
![Figure 4. Delayed mitotic entry upon inhibition of Rac1 and PAK1/2. (A) Synchronized HeLa cells were treated with TcdBF (1000 ng/ml) in G2 phase 8 h after release from the second block. Cell lysates were collected at the indicated time points and were analyzed by western blot analysisusing cell cycle marker proteins. β-actin was used as loading control and Rac1 inactivation by glucosylation as well as PAK phosphorylation, indicative for PAK activity, was determined. (B) Activation of the CyclinB/Cdk1 complex was determined by an in vitro kinase assay. The CyclinB–Cdk1 complex was immunoprecipitated by anti-CyclinB1 antibody and incubated with [γ-32P] ATP in the presence of histone H1, as a Cdk1 substrate. Phosphorylation of histone H1 was determined by autoradiography. (C) Synchronized HeLa cells were transfected with siRNA targeting PAK2 (siPAK2a; siPAK2b), or with scrambled control siRNA (scr). Forty-eight h after transfection, mitotic entry was analyzed in synchronized HeLa cells by western blotting using cell cycle marker proteins. (D) Synchronized Rac1fl/fl MEFs were treated with TcdBF (30 ng/ml) in G2 phase 3 h after release from the second block. Cell lysates were collected at the indicated time points and were analyzed by western blot analysis using cell cycle marker proteins. β-actin was used as loading control and Rac1 inactivation by glucosylation as well as PAK phosphorylation, indicative for PAK activity, was determined. (E) Rac1fl/fl and Rac1−/− MEFs were synchronized by the thymidine double-block technique and analyzed for cell cycle progression by western blot analysis of cell cycle marker proteins.](/cms/asset/322a1606-8a44-46ab-a5ff-3c1c190490a8/kccy_a_10929279_f0004.gif)
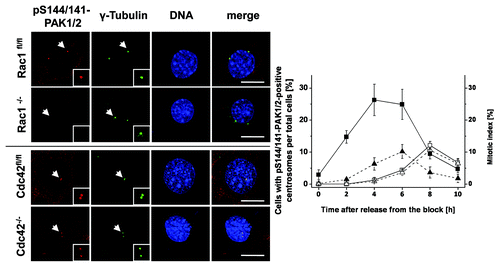
Figure 5. Absence of pS144/141-PAK1/2 from the G2-phase centrosomes of Rac1−/− MEFs. Synchronized Rac1fl/fl and Rac1−/− MEFs and Cdc42fl/- and Cdc42−/− MEFs were synchronized using thymidine double-block technique, fixed at times as indicated and stained for pS144/141-PAK1/2 (red), γ-tubulin (green), and DNA (blue). Scale bar: 15 µm. The number of in Rac1fl/fl (filled symbols) and Rac1−/− (open symbols) MEFs with pS144/141-PAK1/2-positive centrosomes (■) and the mitotic index (▲) were determined by manual counting. Data are mean ± SD from 3 independent experiments with >100 cells in each population.