Figures & data
Figure 1 Subcellular localization of SUMO-1 during mouse oocyte maturation. Oocytes were fixed at 0 h, 4 h, 8 h, 9.5 h and 12 h of culture and then stained with a mouse monoclonal antibody specific for SUMO-1 and counterstained with the fluorescent dye propidium Iodide (PI) to visualize DNA. Green, SUMO-1; red, DNA; GV, oocytes at germinal vesicle stage; Pro-MI, oocytes at first prometaphase; MI, oocytes at first metaphase; ATI, oocytes at first anaphase; MII, oocytes at second metaphase. PB, first polar body; Bar = 20 µm. Experiments were repeated at least three times and representative images are shown.
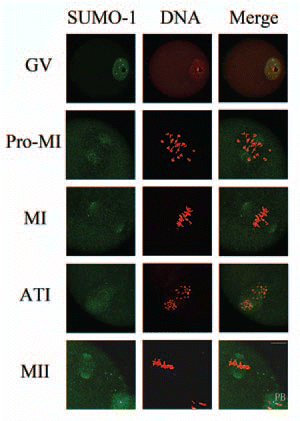
Figure 2 Subcellular localization of SUMO-2/3 during mouse oocyte maturation. Oocytes at different stages (0 h, 2 h, 8 h, 9.5 h and 12 h of culture, respectively) were immunolabeled with a mouse monoclonal antibody specific to SUMO-2/3 (green). GV, oocytes at germinal vesicle stage; GVBD, oocytes after germinal vesicle breakdown; MI, oocytes at first metaphase; ATI, oocytes at first anaphase; MII, oocytes at second metaphase. Each sample was counterstained with PI (red) to visualize DNA. PB, first polar body; Bar = 20 µm. Experiments were repeated at least three times and representative images are shown.
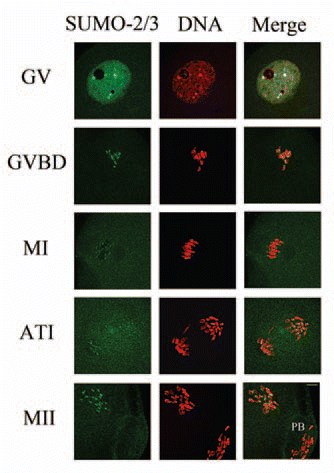
Figure 3 Expression of SUMO-1 and SUMO-2/3 modified proteins during mouse oocyte maturation. Samples were collected after oocyte culture for 0, 4, 8, 9.5 and 12 h, corresponding to the GV, prometaphase I, metaphase I, anaphase I, metaphase II stages, respectively. Proteins from a total of 250 oocytes were loaded for each sample. Experiments were repeated three times.
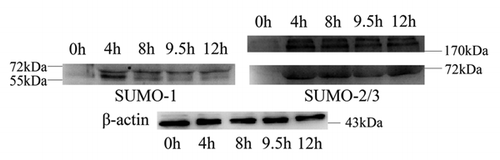
Figure 4 Overexpression of Senp2 resulted in changes of SUMO modified proteins. (A) Myc-Senp2 protein was expressed in mouse oocytes. Myc-Senp2 mRNA microinjected GV oocytes were incubated in M16 medium containing 2.5 µM milrinone for 5 h and collected immediately; the lysates were then analyzed by immunoblot with mouse monoclonal anti-myc antibody. Equal numbers of oocytes microinjected with Myc mRNA was analyzed as a control. (B) Germinal vesicle oocytes were microinjected with the control Myc mRNA and Myc-Senp2 mRNA, respectively. After microinjection, oocytes were incubated in M16 medium with 2.5 µM milrinone for 5 h, then transferred to milrinone-free M16 medium for 10 h and collected, followed by immunoblot analysis with SUMO-1 or SUMO-2/3 specific mouse monoclonal antibodies. Three independent experiments were performed.
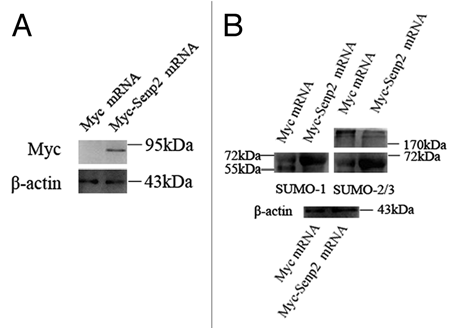
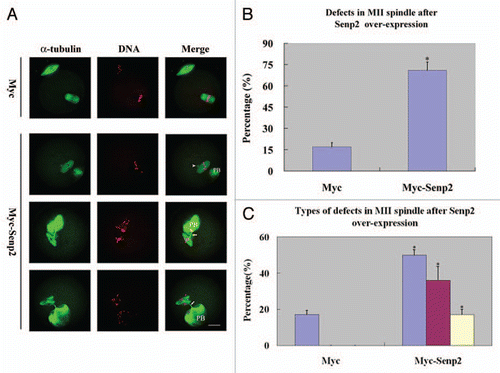
Figure 5 Overexpression of Senp2 impaired spindle organization in MII mature eggs. (A) MII spindle morphologies in Myc mRNA and Myc-Senp2 mRNA microinjection groups. After microinjection, GV oocytes were incubated in M16 medium with 2.5 µM milrinone for 5 h, then transferred to milrinone-free M16 medium for 14 h to fully mature into MII eggs and fixed immediately, followed by immunoflurescent analysis with anti-α-tubulin-FITC antibody (green) and PI (red). In the Myc mRNA microinjection group, normal MII spindles formed. In the Myc-Senp2 mRNA microinjection group, several forms of morphologically aberrant MII spindles were seen in mature eggs. Arrow head, multipolar spindle; arrows, microtubule bundles connecting MII spindles and the first polar bodies. PB, first polar body, Bar = 20 µm. Three independent experiments were performed and representative images are shown. (B) The rate of abnormal MII spindles was recorded in the Myc mRNA microinjection group and the Myc-Senp2 mRNA microinjection group. Data are presented as mean percentage (mean ± SEM) from three independent experiments *p < 0.05. (C) The rates of the main forms of defects in MII spindles were recorded in the Myc mRNA microinjection group and the Myc-Senp2 mRNA microinjection group. Blue: MII spindles with defective morphology; purple: MII spindles with branched microtubule bundles connecting the first polar body; Yellow: MII spindles with both of the above defects. Data are presented as mean percentage (mean ± SEM) from three independent experiments *p < 0.05.