Figures & data
Figure 1 Response of Brca1Δ5–13/Δ5–13;p53Δ2–10/Δ2–10 tumors to dose-dense cisplatin treatment. (A) Example of 2 animals carrying an orthotopically transplanted Brca1Δ5–13/Δ5–13;p53Δ2–10/Δ2–10 tumor (in this case T1). When the tumor reached a volume of 200 mm3 animals were treated with 6 mg cisplatin per kg i.v. on day 0 (blue) or on days 0 and 14 (green). The same treatments were resumed when tumors grew to 100% of their original size (indicated by blue rhombi and green squares). After 6 doses of cisplatin, animals had to be sacrificed due to kidney failure. The growth of the untreated control is shown in pink. (B) Relapse-free survival of 31 animals treated as outlined in (A). Animals are carrying 18 (blue, cisplatin day 0) or 13 (green, cisplatin days 0 and 14) individual orthotopically transplanted Brca1Δ5–13/Δ5–13;p53Δ2–10/Δ2–10 tumors. p value was determined using the Wilcoxon test.
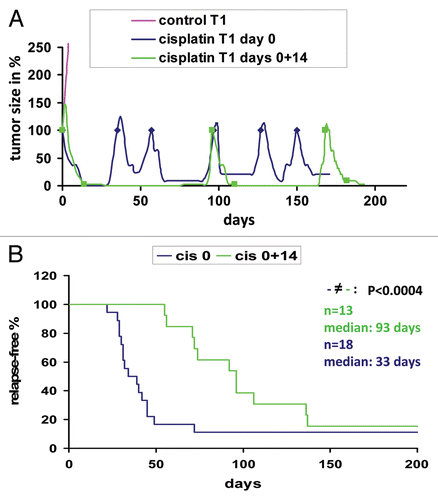
Figure 2 Fractionation of Brca1Δ5–13/Δ5–13;p53Δ2–10/Δ2–10 tumors using FACS. (A) Sorting of live (propidium iodide-negative), Lin- (non-endothelial, non-fibroblastic and non-hematopoietic) cells with CD24- and CD49f-specific antibodies. (B) Tumorigenicity of limiting dilutions of sorted fractions after orthotopic transplantation into syngeneic animals. (C) The FACS-sorted Lin−/CD24+/CD49f+ tumor fraction was collected (left) and re-analyzed by FACS (right) to verify the purity of the collected fraction.
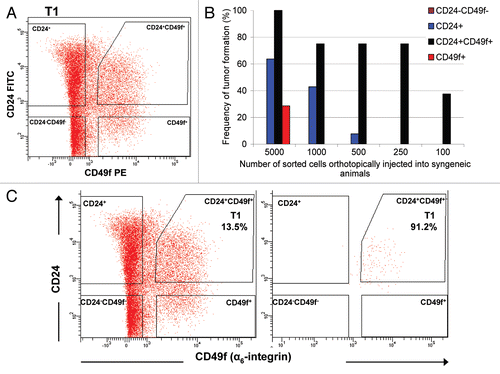
Figure 3 Enhanced tumorsphere-forming ability of Lin−/CD24+/CD49f+ cells in comparison with other sorted subpopulations. (A) Morphology of tumorspheres grown in ultra-low attachment plates from Lin−/CD24+/CD49f+ fraction and other sorted cells in culture. Lin−/CD24+/CD49f− cells occasionally formed small cell aggregates but did not generate tumorspheres. Images were taken on day 10 after plating of freshly sorted cells. Bars represent 50 µm. (B) Quantification of tumorsphere-initiating cells based on data generated from culturing sorted cells under non-adherent conditions from three independent sorted tumors. (C) FACS profile of a tumor generated upon orthotopic transplantation of 50 CD24+/CD49f+ spheres into a syngeneic female mouse.
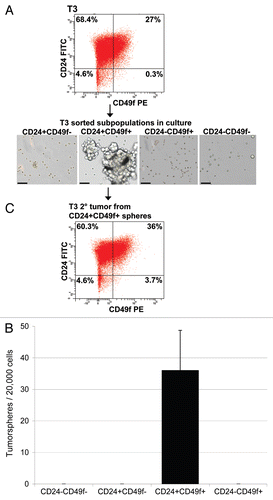
Figure 4 Characteristics of subpopulation-generated tumors compared to the donor tumor. (A) Time to tumor outgrowth upon transplantation of sorted subpopulations based on a combination of Lin−, CD24 and CD49f markers. Graph depicts time to tumor incidence for tumors that grew from 17 Lin−/CD24+/CD49f−, 32 Lin−/CD24+/CD49f+ and 2 Lin−/CD24−/CD49f+ subpopulations. Statistical analysis performed to determine any differences among the three groups was one-way analysis of variance (ANOVA). (B–M) Histological profiling by immunostaining for stromal markers vimentin (Vim) and smooth muscle actin (SMA) and the tumor-initiating cell marker, CD44. The parts show tumor sections from parental tumor T2 (B, F and J) and tumors resulting from the inoculation of 1,000 T2 Lin−/CD24+/CD49f− cells (C, G and K), 1,000 T2 Lin−/CD24+/CD49f+ cells (D, H and L) and 5,000 T2 Lin−/CD24−/CD49f+ cells (E, I and M). Bar represents 50 µm.
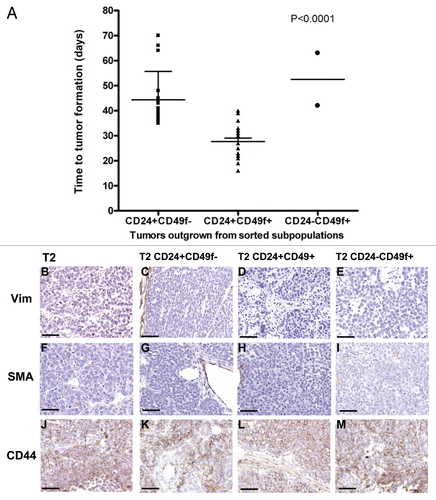
Figure 5 Histological and FACS profiles of subpopulation-derived tumors compared to the donor tumor. Donor tumor T2 (A, E, I, M and Q) and tumors resulting from orthotopic transplantation of 1,000 T2 Lin−/CD24+/CD49f− cells (B, F, J, N and R), 1,000 T2 Lin−/CD24+/CD49f+ cells (C, G, K, O and S) or 5,000 T2 Lin−/CD24−/CD49f+ cells (D, H, L, P and T) are shown. Single color controls (Lin-Cy5, CD24-FITC, CD49f-PE or PI) were included in each FACS experiment, with compensation done by the software. Bars represent 50 µm.
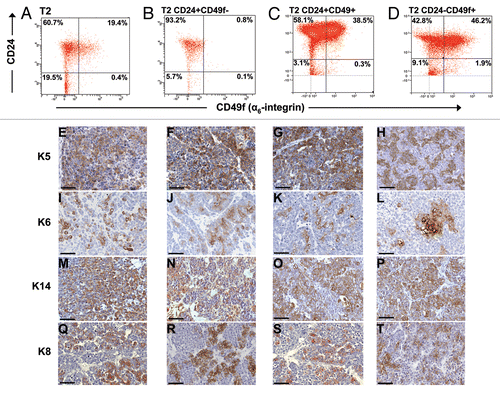
Figure 6 Lin−/CD24+/CD49f+ and Lin−/CD24+/CD49f− fractions repeatedly form tumors after multiple consecutive rounds of sorting and transplantation. (A) FACS plot of tumor T3 grown from spheres and subsequent outgrown tumors from orthotopically transplanted Lin−/CD24+/CD49f− and Lin−/CD24+/CD49f+ cell fractions. Tumors were analyzed on different days. In compliance with Alexander et al.Citation49 single color controls (Lin-Cy5, CD24-FITC, CD49f-PE or PI) were included in each experiment and the compensation was done by the software. (B) Unsupervised hierarchical cluster analysis of gene expression data from tumors outgrown after sorting. The populations used for orthotopic transplantation are indicated in parenthesis.
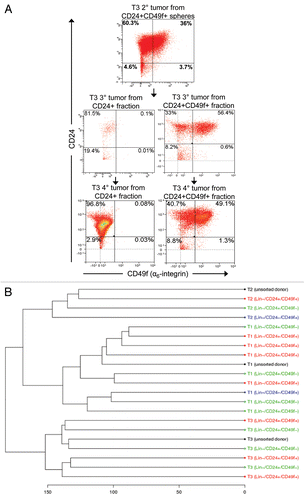
Figure 7 Tumor-initiating cells are not enriched in the tumor remnants that survive cisplatin treatment. FACS analyses of cells dissociated from untreated tumors and tumor remnants 10 days after i.v. injection of 6 mg cisplatin (CDDP) per kg, represented graphically in (A) and summarized in . (B) Repeated cisplatin sensitivity of tumors generated from sorted cells in comparison with the donor tumor. Arrows indicate cisplatin administration (6 mg/kg i.v.) after each tumor reached a size that was 100% of the initial tumor. (C) The same analysis as in (A), using a CD29 antibody instead of CD49f.
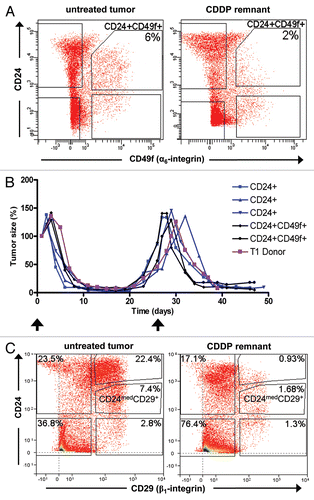
Table 1 Tumor incidence summary of the graph shown in
Table 2 Lin−/CD24+/CD49f+ and Lin−/CD24+/CD49f− fractions repeatedly form tumors after multiple consecutive rounds of sorting and transplantation
Table 3 Summary of FACS analyses of cells dissociated from untreated tumors and tumor remnants 10 days after i.v. injection of 6mg cisplatin (CDDP) per kg