Figures & data
Figure 1 Schematic representation of the isolation and genetic characteristics of two plasma cell lines isolated from the same patient. The ALMC-1 cell line was established at the time of initial presentation with cardiac AL amyloidosis. After two months of therapy with dexamethasone, the patient underwent peripheral blood stem cell transplantation (PBSCT) with melphalan conditioning. Three months later, the patient was diagnosed with multiple myeloma and the ALMC-2 cell line established. Characteristic cytogenetic abnormalities of both cell lines are represented including shared and unique cytogenetic abnormalities. The karyotype obtained from the bone marrow biopsy three months after the stem cell transplant is also included (middle part). The primary MM cells at this time point were near diploid.
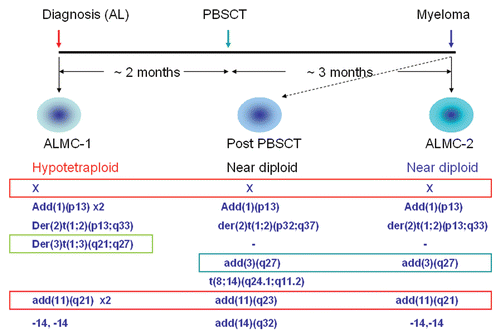
Figure 2 Population kinetics under cytokine stimulation. The population size of both ALMC-1 (top part) and ALMC-2 cells (bottom part) growing in the presence or absence of growth factor stimulation were determined daily. In (A) we compare growth of the two populations in the presence of bovine serum albumin (BSA) or BSA supplemented with IL-6 and IGF-1 (denoted by ++). In (B) we compare population growth in the presence of fetal calf serum (FCS) with or without IL-6 and IGF-1 (denoted by ++). Addition of both cytokines enhanced growth of ALMC-2 while slowing growth of ALMC-1.
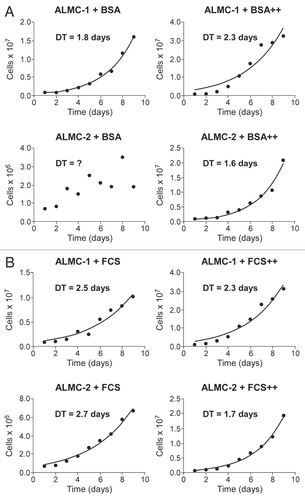
Figure 3 Mathematical model of cell dynamics for both cell lines. Whenever a cell divides, with probability p, chromosome segregation and cytokinesis occur normally, while with probability 1 − p, chromosome duplication is not followed by cytoplasmic division giving rise to a cell with doubling of ploidy.
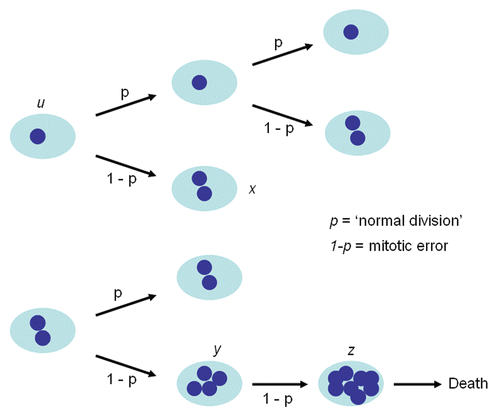
Figure 4 Population composition and growth kinetics. The dynamics of the two populations (•) go beyond simple exponential growth due to the presence of various subpopulations with different ploidy. In order to determine the value of p and the population doubling times, we fitted our model to experimental growth data using a non-linear least squares method. We imposed the experimentally determined values of the fraction of cells with higher ploidy at specific time intervals (y-axes of inset) obtained using FISH for ALMC-1 (C: (▾) near tetraploid fraction, (♦) near octaploid fraction) and ALMC-2 (D: (▪) near diploid fraction, (▾) near tetraploid fraction and (♦) near octaploid fraction) respectively. The continuous lines are the result of the fits.
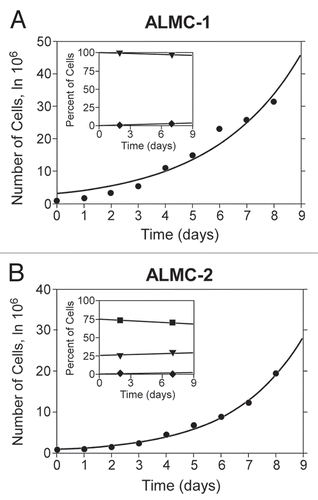
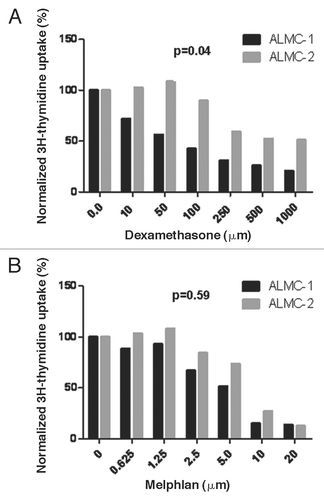
Figure 5 In vitro sensitivity to therapeutic agents used in the patient. The replication rates of ALMC-1 and ALMC-2 cells in the presence of varying concentrations of dexamethasone (A) and melphalan (B) were determined by 3H-thymidine uptake. ALMC-1 cells are more sensitive to dexamethasone while both cell lines are equally sensitive to melphalan.