Figures & data
Figure 1 E2F-1 is degraded in late S/G2 phase. (A and B) HeLa cells were synchronized in prometaphase with 330 nM nocodazole for 16 h, collected and replated in fresh medium. Cells were harvested at the indicated time points and processed for immunoblotting (A) or FACS analysis (B). (C and D) HeLa cells were synchronized at G1/S using a double thymidine block as described in Materials and Methods. Following release from the double thymidine block, cells were harvested at the indicated time points and processed for immunoblotting (C) or FACS analysis (D).
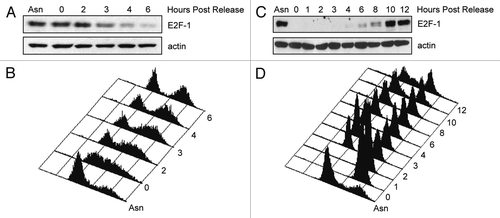
Figure 2 Cdc20 and Cdh1 reduce ectopic E2F-1 levels. (A) HeLa cells were transfected with HA-tagged E2F-1 alone and in the presence of HA-tagged Cdc20, HA-tagged Cdh1, FLAG-tagged β-Trcp1 or FLAG-tagged Skp2 at ratios of 1:5 and 1:15 (E2F1 and each ubiquitin ligase). All transfections were balanced with empty vector (EV, pcDNA3). Forty-eight hours after transfection, cells were harvested and extracts analyzed by immunoblotting with anti-HA, anti-FLAG or anti-actin antibodies. (B) T98G cells were transfected and analyzed as in (A). (C) HeLa cells were transfected with HA-tagged E2F-1 in the presence of HA-tagged Cdc20 (left part) or HA-tagged Cdh1 (right part) at a ratio of 1:15. Forty-eight hours after transfection, cells were treated with 100 µg/mL cycloheximide (CHX), collected at the indicated time points and extracts were analyzed by immunoblotting with anti-HA or anti-actin antibodies. Densitometric analysis was performed on E2F1 protein normalized to actin using Kodak 1D Image Analysis software. E2F1 alone was quantified using the lighter exposure and E2F1 co-expressed with Cdc20 or Cdh1 was quantified from the dark exposure.
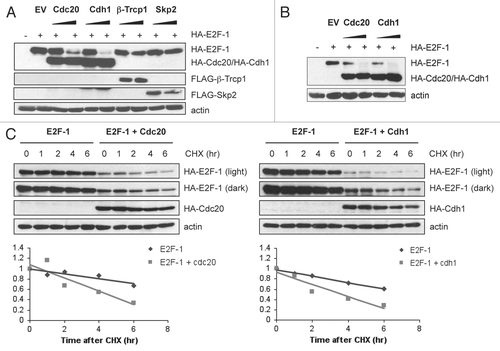
Figure 3 DP1, but not Rb, partially protects E2F-1 from APC/C-mediated degradation. (A) HeLa cells were transfected with HA-tagged E2F-1 (100 ng) in the presence of HA-tagged Cdc20 (1.5 µg), HA-tagged Cdh1 (1.5 µg), HA-tagged DP1 (100 ng) or pCMV-pRb (400 ng) as indicated. All transfections were balanced with empty vector (pcDNA3). 48 h after transfection, cells were harvested and extracts analyzed by immunoblotting.
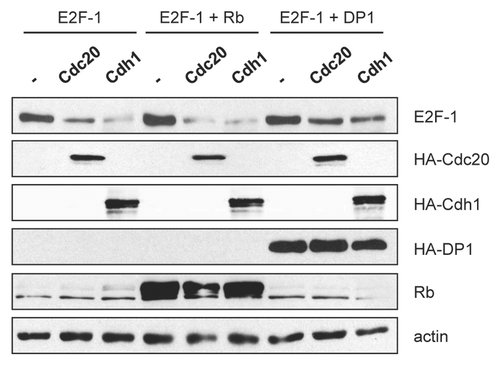
Figure 4 E2F1 interacts with Cdc20 and Cdh1. (A) HeLa cells were transfected with constructs encoding HA-tagged E2F1, FLAG-tagged Cdh1, Cdc20 or Cdc20 ΔN165 as indicated at a ratio of 1:1.5 (E2F1 and each ubiquitin ligase). All transfections were balanced with empty vector (pcDNA3). 24 h after transfection, cells were harvested and extracts were prepared. Equal amounts of protein were subjected to immunoprecipitation with anti-FLAG antibody as described in Materials and Methods, then analyzed by immunoblotting with anti-HA antibody. 10% of the lysate was used for the input samples. (B) HeLa cells were transfected with HA-tagged E2F-1 (100 ng) with increasing amounts of FLAG-tagged Cdc20 (wild-type), Cdc20 ΔN165 or Cdh1 (0, 0.5, 1 or 2 µg). All transfections were balanced with empty vector (pcDNA3). Forty-eight hours after transfection, cells were harvested and extracts analyzed by immunoblotting with anti-HA or anti-FLAG antibodies. (C) Alignment of the amino acid regions corresponding to the putative destruction box motifs (Dbox1 and Dbox2) in E2F1 with the D box motifs of cdc6, cyclin B1, securin, cyclin A, geminin, Skp2, Plk1 and p21. (D) HeLa cells were transfected with HA-tagged E2F-1 wild-type (wt) (100 ng), E2F-1 ΔDbox1 (100 ng), E2F-1 ΔDbox2 (100 ng) or E2F-1 ΔDbox1 + 2 (100 ng) alone or in the presence of HA-tagged Cdc20 (1.5 µg) or HA-tagged Cdh1 (1.5 µg). All transfections were balanced with empty vector (pcDNA3). Forty-eight hours after transfection, cells were harvested and extracts analyzed by immunoblotting with anti-HA or anti-actin antibodies.
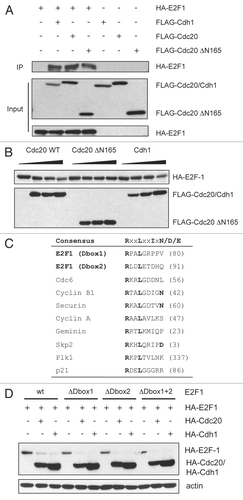
Figure 5 E2F1 is degraded in a Cdc20-dependent manner in vivo. (A) HeLa cells were transfected with control (Luc), Cdh1 or Cdc20 siRNA oligos. Forty-eight hours after transfection, cells were harvested and extracts analyzed by immunoblotting with antibodies to the indicated proteins. (B) Extracts prepared from cells treated as in (A) were supplemented with a degradation cocktail as described in Materials and Methods, incubated at room temperature and harvested at the indicated times. Levels of E2F1, cyclin B and actin were analyzed by immunoblotting. (C) E2F1 is hyper-ubiquitinated in cells. Extracts from H1299 cells transfected with Myc-E2F1 (100 ng), HA-Ubiquitin (500 ng), Flag-Cdc20 (50 and 100 ng) and Flag-Cdc20 ΔN (100 ng) as indicated, were immunoprecipitated (IP) with anti-Myc antibody as described in Materials and Methods. Ubiquitin conjugates were detected by immunoblotting IP samples with anti-HA antibody. Expression levels of transfected proteins were detected by western blotting shown in the right 3 parts (WCL).
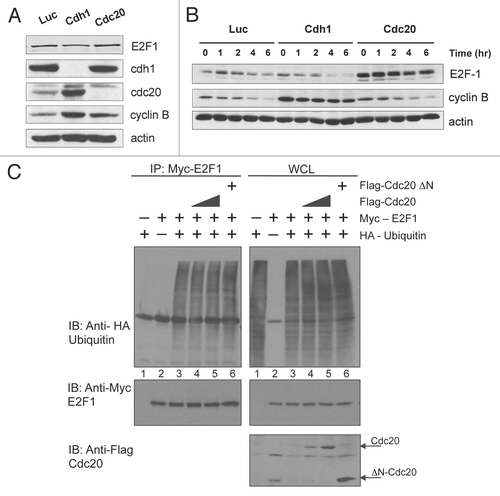
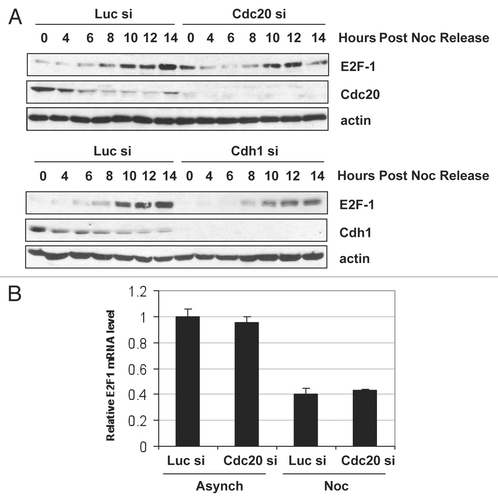
Figure 6 Cdc20 targets E2F1 for degradation in prometaphase. (A) HeLa cells were transfected with control (Luc), Cdc20 or Cdh1 siRNA oligos. 32 h later, fresh medium was added and cells were treated with 330 nM nocodazole for an additional 16 h to arrest cells in prometaphase. Cells were then replated in fresh media and harvested at the indicated times after release. Extracts were prepared and analyzed by immunoblotting with antibodies to the indicated proteins. (B) HeLa cells were transfected with control (Luc) or Cdc20 siRNA oligos. 32 h later, fresh medium was added and cells were left untreated (asynchronous) or treated with 330 nM nocodazole (Noc) for an additional 16 h to arrest cells in prometaphase. Total RNA was isolated and quantitative RT-PCR analysis was performed. Relative E2F1 mRNA levels were calculated by normalizing to L32 and data are representative of three independent experiments.