Figures & data
Figure 1 Model for nuclear polyadenylation/deadenylation machineries involved in RNA surveillance and DDR. (A) In yeast, the TRAMP complex-mediated polyadenylation/deadenylation is mechanistically implicated in mRNA surveillance. The TRAMP complex and the exosome are recruited to defective RNAs at sites of active transcription by RNAP II. The TRAMP complex, which contains Trf4p and Trf5p (noncanonical PAPs), Mtr4p (RNA helicase) and Air1p/Air2p (RNA binding protein), adds a ∼40 nucleotides tail to the 3′ end of target RNA to mark it for degradation by the exosome. The CCR4-NOT deadenylase can interact physically and functionally with both the exosome and the TRAMP complex. Rrp6, a nuclear exosome component, is necessary not only for RNA exonucleolytic degradation but also for transcription termination. (B) In mammalian cells, the nuclear polyadenylation/deadenylation switch regulates RNA turnover in response to DNA damage. In the absence of DNA damage treatment, CBP80 binds to nuclear deadenylase PARN and inhibits its deadenylase activity. The elongating RNAP II-CstF holoenzyme is active and polyadenylation takes place generating normal levels of mRNA. Upon UV-induced DNA damage, PARN dissociates from CBP80 and interacts with the CstF1/BARD1 complex. Interestingly, the formation of PARN/CstF1/BARD1 complex not only inhibits RNA 3′ end cleavage, but at the same time also activates the deadenylation by PARN and leads to RNA degradation.
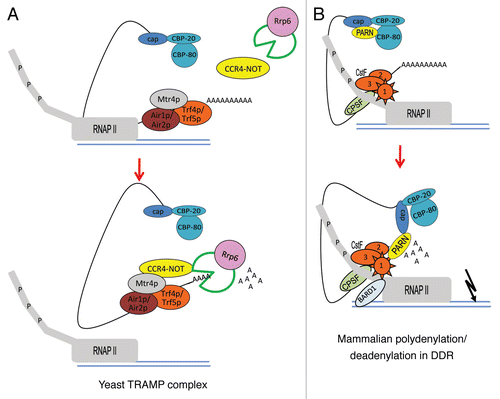
Figure 2 Model for cytoplasmic polyadenylation/deadenylation during cell differentiation processes. (A) The deadenylation and polyadenylation of dormant mRNAs are controlled by cis-elements at 3′UTR, such as the CPE and the AAUAAA signals, which are recognized by the polyadenylation factors CPEB and CPSF, respectively. Poly(A) polymerase Gld2 and deadenylase PARN are crucial components of CPEB-containing complexes in the regulation of deadenylation/polyadenylation. After nuclear polyadenylated mRNAs are transported to the cytoplasm, their poly(A) tails are shortened by PARN deadenylase associated to CPEB-containing complexes. Although Gld2 polymerase is also associated to CPEB-containing complexes, its activity is inhibited. During meiotic cell cycle progression, CPEB is phosphorylated and PARN dissociates from CPEB-containing complex, allowing Gld2 to elongate the poly(A) tail and to label the CPE containing mRNAs for translation. (B) Model for translation repression by deadenylation. As deadenylation is the first step in this highly regulated process of cytoplasmic polyadenylation/deadenylation during meiosis, other deadenylation factors are also involved allowing a strict spatial and temporal regulation. Many CPE containing mRNAs also contain other signals in their 3′UTR, such as Pumilio and/or embryonic deadenylation element (EDEN) binding sites, which are recognized by the deadenylation factors Pumilio and EDEN-BP, respectively. While Pumilio interacts with the conserved deadenylase complex CCR4-POP2-NOT (shown to the left), EDEN-BP interacts with PARN to deadenylate mRNAs (shown to the right). Some of these cis-acting RNA sequences are present in the 3′UTR of the same mRNAs, allowing numerous alternative mechanisms to regulate deadenylation/polyadenylation of mRNAs.
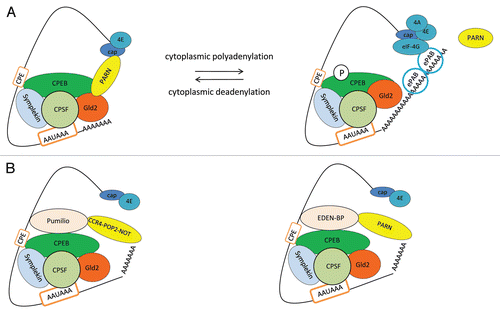
Figure 3 Schematic diagrams of cis-acting RNA sequences at the 3′UTR involved in polyadenylation/deadenylation processes. The selection between the proximal or distal alternative polyadenylation signals leads to the exclusion or inclusion of cis-acting RNA sequences, such as miRNA target sites and ARE, that could mediate polyadenylation/deadenylation processes.
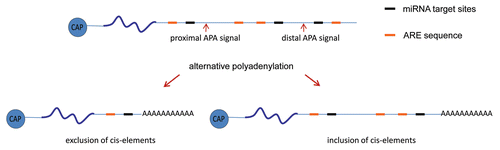
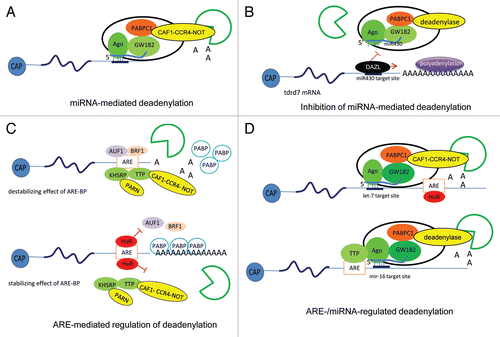
Figure 4 Models of multicomponent complexes required for regulation of polyadenylation/deadenylation processes. (A) miRNA-mediated deadenylation. miRISCs, which contain Ago, GW182, PABPC1 and either CAF1-CCR4-NOT (as indicated) or Pan2-Pan3 (not shown) deadenylases, deliver miRNAs to the target mRNAs and mediate deadenylation, which leads subsequently to mRNA degradation. (B) Inhibition of miRNA-mediated deadenylation. The RNA-binding protein DAZL overcomes the inhibitory effect of miRNA by inducing polyadenylation and leading to active translation even in the presence of miRNA. (C) ARE-mediated regulation of deadenylation. The ARE-binding proteins (BP) mediate destabilization and/or stabilization of the ARE-containing mRNAs. ARE-BPs, such as AUF1, TTP, BRF1 and KHSRP, recruit deadenylases, such as PARN and CAF1-CCR4-NOT, to target ARE-mRNAs and initiate the deadenylation process that precedes degradation. Another ARE-BP, HuR, plays a role in stabilizing ARE-containing mRNAs by blocking the binding of ARE-BPs involved in the destabilization of ARE-mRNAs, such as AUF1, TTP and KHSRP. This competition stabilizes the association of PABP to the poly(A) tail or prevents the recruitment of deadenylases to the ARE-mRNA. (D) ARE- and miRNA-regulated deadenylation. Cooperation of AREs-BPs, miRNAs, deadenylases and exosome is essential for the regulation of mRNA stability. The recruitment of the ARE-BPs HuR or TTP to the ARE sequence assists the targeting of let-7- or miR-16-loaded miRISC complexes, respectively, to the most proximal site to the ARE sequence.