Figures & data
Figure 1 (A) Genotyping. As described in methods, amplification of DNA (100 ng) by PCR yielded fragments of ∼487 bp from PDE3A-/- mice, ∼213 bp from WT mice, and both bands from heterozygous mice. Water was used as a negative control. (B) Western blots of ovarian lysates, performed as described in methods, demonstrated deficiency of immunoreactive PDE3A in PDE3A-/- ovary. (C) Meiotic arrest in PDE3A-/- oocytes. As described in methods, cultured WT oocytes spontaneously resume meiosis in vitro, and, within16 h, extrude the first polar body. PDE3A-/- oocytes remain arrested at GV stage. Oocytes form was captured by a microscopy on objective 20x.
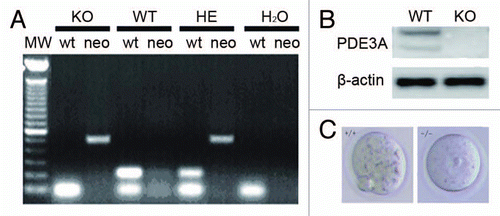
Figure 2 (A and B) PKA activity in cultured oocytes. PKA activity was assayed as described in methods; results are expressed as cpm 32P incorporated into the PKA peptide substrate, Kemptide. (A) In the presence of excess cAMP (2 µM), total PKA activity is similar in lysates from fresh WT and PDE3A-/- oocytes. Basal PKA activity (-cAMP) is significantly increased in PDE3A-/- oocytes, consistent with increased cAMP content in PDE3A-/- oocytes.Citation14 Experiments were repeated three times. Results are presented as m ± SE, n = 3 independent experiments (25 oocytes/assay). (B) Time course of changes in PKA activities: In WT oocytes, PKA activity was reduced for ∼1 h, then increased over the next several hours. In PDE3A-/- oocytes and WT oocytes exposed to cilostamide, PKA activity was elevated during the 5 h incubation. Results are presented as m ± SE, n = 3 independent experiments (25 oocytes/assay). (C) Immunoflurescence of PKA catalytic subunits (PKAc): In freshly isolated WT oocytes, PKAc (green) were detected in the cytoplasm, and some PKAc co-localized with pericentrin (red), presumably on centrosomes. During GVBD, PKAc entered the nucleus and then localized to the spindle apparatus. In PDE3A-/- oocytes, PKAc remained localized in the cytoplasm and on centrosomes, even after incubation for 4 h. n = 3 experiments. Bar, 20 µm.
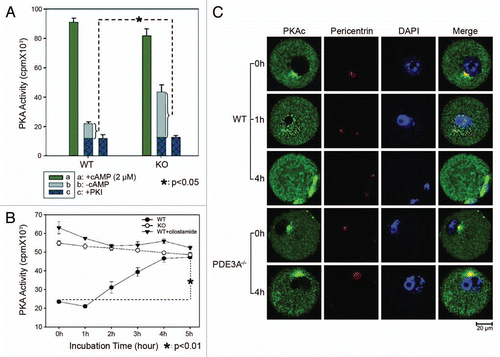
Figure 3 Histone H3 phosphorylation: (A) Oocytes (30 oocytes) were fixed and immunofluoresence performed at antibody dilutions described in methods. In freshly isolated WT oocytes, histone H3 was not phosphorylated at Ser10 (green). In cultured WT oocytes, phosphorylation of histone H3 was initiated within 1 h, and was markedly increased within 4 h, as was histone H3 condensation (an index of chromosomal condensation). In cultured PDE3A-/- oocytes, histone H3 was not phosphorylated at ser10 (green) and chromosome condensation did not occur, even after 18 h.n = 3. (B) At the indicated times, cultured oocytes were harvested, samples (40 WT, 40 PDE3A-/- oocytes) were immediately placed in lysis buffer and lysates were subjected to SDS-PAGE as described in methods. Western Blots demonstrated that after 4 h, histone H3 was phosphorylated at Ser10 in WT oocytes, not in PDE3A-/- oocytes. n = 3 experiments. Bar, 20 µm.
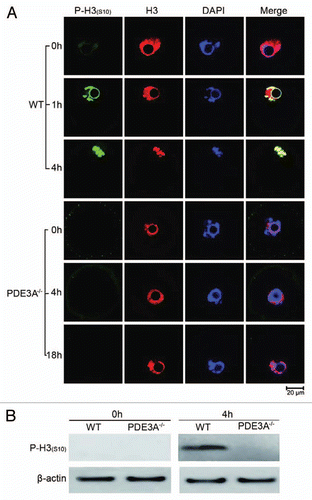
Figure 4 Cdc2 activity and localization: (A) Cultured oocytes were collected at the indicated times, lysates were prepared, and Cdc2 activity was assayed as described in methods. From 1 h to 5 hours, Cdc2 activity significantly increased in cultured WT oocytes, but not in PDE3A-/- oocytes or WT oocytes exposed to cilostamide. Results are expressed as cpm 32P incorporated into the peptide substrate (PKT PKK AKK L) derived from histone H1. Result are presented as m ± SE, n = 3 experiments (25 oocytes/assay at each time point). (B) Western blot. Cultured oocytes (40/time point) were collected at indicated times, and lysates were prepared and subjected to SDS Page/western blots with indicated antibodies as described in methods. Cdc2 was dephosphorylated at T14/Y15 to a much greater extent in WT ooyctes than in PDE3A-/- oocytes; Cdc2 was phosphorylated at Thr161 both in WT and PDE3A-/- oocytes. n = 3 experiments. (C) At the indicated times, cultured oocytes (30 oocytes) were collected and immunoflourescence of Cdc2 was performed as described in methods. In cultured WT oocytes Cdc2 (red) was widely distributed in the cytoplasm and nucleus; during GVBD it localized to the spindle apparatus. Some phospho-Cdc2 [P-Cdc2(T14/Y15)] (green) was localized at MTOCs in fresh WT oocytes, phospho-Cdc2 immunoflouresence was markedly reduced at 4 h and very weak at 18 h. In PDE3A-/- oocytes, phospho-Cdc2 remained in the cytosol, nucleus and at MTOCs. n = 3 experiments. Bar, 20 µm.
![Figure 4 Cdc2 activity and localization: (A) Cultured oocytes were collected at the indicated times, lysates were prepared, and Cdc2 activity was assayed as described in methods. From 1 h to 5 hours, Cdc2 activity significantly increased in cultured WT oocytes, but not in PDE3A-/- oocytes or WT oocytes exposed to cilostamide. Results are expressed as cpm 32P incorporated into the peptide substrate (PKT PKK AKK L) derived from histone H1. Result are presented as m ± SE, n = 3 experiments (25 oocytes/assay at each time point). (B) Western blot. Cultured oocytes (40/time point) were collected at indicated times, and lysates were prepared and subjected to SDS Page/western blots with indicated antibodies as described in methods. Cdc2 was dephosphorylated at T14/Y15 to a much greater extent in WT ooyctes than in PDE3A-/- oocytes; Cdc2 was phosphorylated at Thr161 both in WT and PDE3A-/- oocytes. n = 3 experiments. (C) At the indicated times, cultured oocytes (30 oocytes) were collected and immunoflourescence of Cdc2 was performed as described in methods. In cultured WT oocytes Cdc2 (red) was widely distributed in the cytoplasm and nucleus; during GVBD it localized to the spindle apparatus. Some phospho-Cdc2 [P-Cdc2(T14/Y15)] (green) was localized at MTOCs in fresh WT oocytes, phospho-Cdc2 immunoflouresence was markedly reduced at 4 h and very weak at 18 h. In PDE3A-/- oocytes, phospho-Cdc2 remained in the cytosol, nucleus and at MTOCs. n = 3 experiments. Bar, 20 µm.](/cms/asset/1988a3a3-b403-40ae-9aea-463481446097/kccy_a_10914090_f0004.gif)
Figure 5 (A) Immunoflourescence of Cyclin B1 in cultured oocytes (30 oocytes each time point) was performed as described in methods. In fresh WT oocytes, Cyclin B1 signal (green) was primarily in the cytoplasm, with some localization at MTOCs. Within 1 h, Cyclin B1 immunofluoresence increased in nucleus, and within 4 h, localized to the spindle apparatus and centrosomes. In cultured PDE3A-/- oocytes, Cyclin B1 localization did not change, even after 4 h incubation. n = 3 experiments. (B) Western blots. At the indicated times, cultured oocytes (40 oocytes/time point) were collected, and lysates were prepared and subjected to SDS PAGE/western blots as described in methods. Immunoreactive Cyclin B1 seems to transiently increase and then decrease in cultured PDE3A-/- oocytes. n = 3 experiments. Bar, 20 µm.
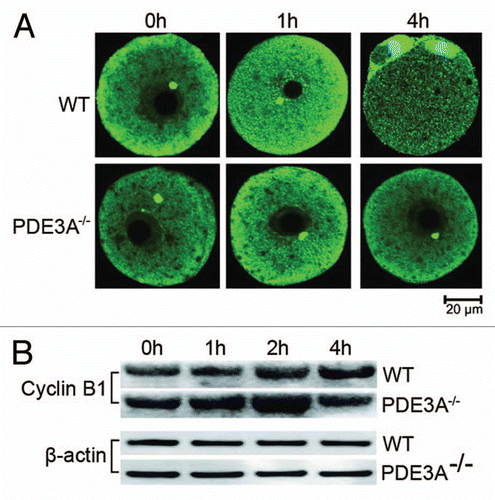
Figure 6 (A) Immunoflourescence of Cdc25B in cultured oocytes. At indicated times, cultured oocytes (30 oocytes each time point) were collected, fixed and immunofluorescence of Cdc25B (green) assessed as described in methods. In freshly prepared WT and PDE3A-/- oocytes, immunoreactive Cdc25B (Green) was relatively more highly expressed in nucleus than in cytoplasm. Within 1 h, nuclear Cdc25B was decreased in cultured WT, not PDE3A-/-, oocytes. After 4 h, Cdc25B immunofluoresence was markedly decreased in GVBD WT oocytes as compared to cultured PDE3A-/- oocytes, in which nuclear Cdc25B was relatively stable during the 4 h incubation. In the 4 h WT panel, the chromatin at the bottom left is most likely from accompanying cumulus cells. n = 3 experiments. (B) Western blots of phospho-Cdc25B. At indicated times, cultured oocytes were collected (40 oocytes/time point), and lysates were prepared and subjected to SDS PAGE/western blots, using anti-phospho-Cdc25B (Ser323), as described in methods. Cdc25B was dephosphorylated at Ser323 within 1 h in cultured WT oocytes, but not in PDE3A-/- oocytes. n = 3 experiments. Bar, 20 µm.
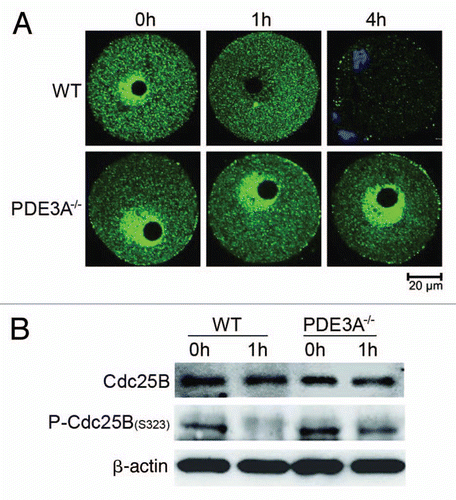
Figure 7 Wee1 and Check kinase activities in cultured oocytes. At indicated times, cultured WT and PDE3A-/- oocytes were collected (100 oocytes/time point), lysates prepared, and enzymatic assays performed as described in methods. Results are presented as m ± SE, n = 3 experiments. (A) Time course of Wee1 activity: Wee1 activity (expressed as the color quantitated by spectrophotometry at 450 nm) decreased in cultured PDE3A-/- and WT oocytes, and WT oocytes exposed to cilostamide. (B) Check kinase activity: There are no significant differences in checkpoint kinase activity (expressed as the color quantitated by spectrophotometry at 450 nm) between cultured WT and PDE3A-/- oocytes.
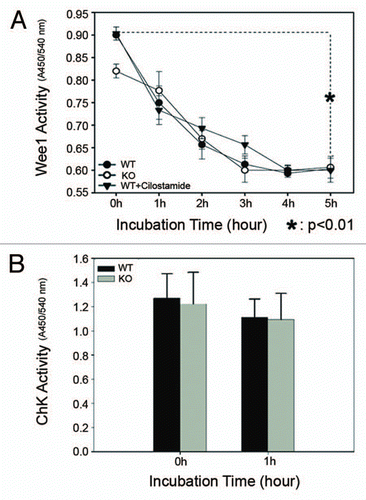
Figure 8 Western blot of key proteins for G2/M transition. As described in methods, lysates from freshly prepared WT and PDE3A-/- oocytes (40 oocytes) were subjected to SDS-PAGE/western Blot. Membranes were incubated with indicated primary antibodies at 4°C overnight, followed with second antibody for 1 h at room temperature.
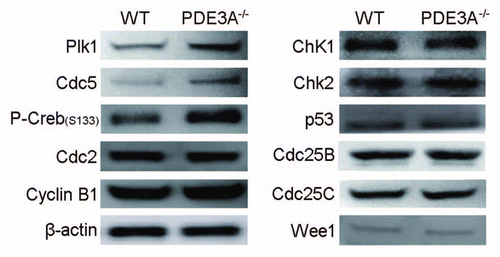
Figure 9 (A) Plk1 activity in cultured oocytes. Cultured oocytes (100 oocytes per time point) were collected at the indicated times to assess Plk1 activity. Some WT oocytes (100 oocytes) were treated with cilostamide (10 µM) for 4 h, and some PDE3A-/- oocytes (100 oocytes) were treated with the PKA inhibitor, Rp-cAMP (5 mM), for 2 h and then incubated in the absence of Rp-cAMP for 2 h before Plk1 assay. Plk1 was immunprecipitated from oocyte lysates. Plk1 activity was assayed by incubation of immunoprecipitated Plk1 with 32P-ATP and α-casein substrate as described in methods. After Phosphor Imager analysis of wet gels to measure 32P-labelled α-casein, SDS PAGE gels were electro-transferred to membranes for western blots for detection of immunoreactive oocyte Plk1. n = 3 experiments. (B) Phosphorylation/inactivation of rPlk1 by purified PKAc in vitro. As described in methods, rPlk1 was incubated with indicated units of purified PKAc and 32P-ATP at 30°C for 15 min; PKI (4 µM) was added to terminate the reaction. To half volume of the mixture, dephosphorylated α-casein substrate (0.5 mg/ml) was added, to assess Plk1 activity i.e., Plk1-induced phosphorylation of α-casein. The other half volume was used for Plk1 immunoprecipitation to check PKAc-induced phosphorylation of rPlk1. Results showed that purified PKAc phosphorylated rPlk1 in vitro and inhibited its activity. n = 5 experiments. (C) PDE3A co-immunoprecipitates with Plk1 in mice ovary and Hela cells. As described in methods, total lysates were prepared from WT mouse ovaries (to increase the amount of material available) (0.6 mg/IP) and Hela cells (2.0 mg/IP) and incubated with anti-PDE3A or anti-Plk1 antibodies or non-immine IgG. Immunoprecipitates were subjected to SDS PAGE and western blots, using the indicated primary antibodies. PDE3A and Plk1 co-immunoprecipitated in lysates from mouse ovary and Hela cells. n = 3 experiments. (D) PKA inhibited endogenous Plk1 activity in vitro and Plk1 interacted with PDE3A-PKAc macromolecular complex in Hela cells. (Upper part) Endogenous Plk1 was immunoprecipitated from Hela cells (1 mg/IP), followed by incubation of immunoprecipitated Plk1 (beads) with indicated units of purified PKAc and 32P-ATP at 30°C for 15 min; PKI (4 µM) was added to terminate the reaction. PKAc-induced phosphorylation of Hela cell Plk1 and inhibition of Plk1-induced phosphorylation of “-casein substrate were analyzed as described in methods and in legend to (B). (Lower part): Hela cells were homogenized in ice-cold buffer A [50 mM Hepes, 1 mM EDTA, 10 mM pyrophosphate, 5 mM MgCl2, 5 mM NaF, 100 mM NaCl, 0.1 µM okadaic acid, phosphatase inhibitor cocktail (Calbiochem) and Roche protease inhibitor cocktail (pH 7.5)] with dounce homogenizer (on ice, 20 strokes), sonicated (on ice, 20 pulses, 40% duty cycle, output scale 4), and solubilized with 1% NP-40 (Calbiochem) (1% final). Samples were cleared by incubation (1 h) with 5 µg non-immune IgG, and then with Dynabeads protein G (Invitrogen, Cat# 100.04D) for 30 min before centrifugation (2,800 g, 4°C, 5 min). Cleared lysates from Hela cells (2 mg/IP) were incubated (overnight, 4°C) with mouse anti-Plk1 (10 µg/IP) (Millipore, Cat# 05-844) and non-immune mouse IgG (10 µg/IP), followed by incubation (1 h) with fresh Dynabeads protein G as described in methods. Immunoblot data indicated that Plk1 co-immunoprecipitated with PDE3A, PKA-C, PP2A and Cdc25C. n = 3 experiments. (E) Subcelluar localization of Plk1 and PDE3A in WT and PDE3A-/- oocytes. As described in methods, at the indicated times, cultured oocytes were collected, fixed, and assessed for immunofluorescence of PDE3A and Plk1. In freshly prepared WT oocytes, Plk1 was detected in the cytoplasm, with some localization at MTOCs. Within 1 hour, Plk1 migrated into the nucleus, and at GVBD, it was detected at MTOCs and kinetochores. In cultured WT oocytes PDE3A was partly co-localized with Plk1 at the spindle apparatus. In cultured PDE3A-/- oocytes, even after 4 hours, Plk1 immunofluorescence was only detected in the cytoplasm, with some localization at MTOCs, but not in the nucleus. No PDE3A signal was detected in PDE3A-/- oocytes. n = 3 experiments. Bar, 20 µm.
![Figure 9 (A) Plk1 activity in cultured oocytes. Cultured oocytes (100 oocytes per time point) were collected at the indicated times to assess Plk1 activity. Some WT oocytes (100 oocytes) were treated with cilostamide (10 µM) for 4 h, and some PDE3A-/- oocytes (100 oocytes) were treated with the PKA inhibitor, Rp-cAMP (5 mM), for 2 h and then incubated in the absence of Rp-cAMP for 2 h before Plk1 assay. Plk1 was immunprecipitated from oocyte lysates. Plk1 activity was assayed by incubation of immunoprecipitated Plk1 with 32P-ATP and α-casein substrate as described in methods. After Phosphor Imager analysis of wet gels to measure 32P-labelled α-casein, SDS PAGE gels were electro-transferred to membranes for western blots for detection of immunoreactive oocyte Plk1. n = 3 experiments. (B) Phosphorylation/inactivation of rPlk1 by purified PKAc in vitro. As described in methods, rPlk1 was incubated with indicated units of purified PKAc and 32P-ATP at 30°C for 15 min; PKI (4 µM) was added to terminate the reaction. To half volume of the mixture, dephosphorylated α-casein substrate (0.5 mg/ml) was added, to assess Plk1 activity i.e., Plk1-induced phosphorylation of α-casein. The other half volume was used for Plk1 immunoprecipitation to check PKAc-induced phosphorylation of rPlk1. Results showed that purified PKAc phosphorylated rPlk1 in vitro and inhibited its activity. n = 5 experiments. (C) PDE3A co-immunoprecipitates with Plk1 in mice ovary and Hela cells. As described in methods, total lysates were prepared from WT mouse ovaries (to increase the amount of material available) (0.6 mg/IP) and Hela cells (2.0 mg/IP) and incubated with anti-PDE3A or anti-Plk1 antibodies or non-immine IgG. Immunoprecipitates were subjected to SDS PAGE and western blots, using the indicated primary antibodies. PDE3A and Plk1 co-immunoprecipitated in lysates from mouse ovary and Hela cells. n = 3 experiments. (D) PKA inhibited endogenous Plk1 activity in vitro and Plk1 interacted with PDE3A-PKAc macromolecular complex in Hela cells. (Upper part) Endogenous Plk1 was immunoprecipitated from Hela cells (1 mg/IP), followed by incubation of immunoprecipitated Plk1 (beads) with indicated units of purified PKAc and 32P-ATP at 30°C for 15 min; PKI (4 µM) was added to terminate the reaction. PKAc-induced phosphorylation of Hela cell Plk1 and inhibition of Plk1-induced phosphorylation of “-casein substrate were analyzed as described in methods and in legend to (B). (Lower part): Hela cells were homogenized in ice-cold buffer A [50 mM Hepes, 1 mM EDTA, 10 mM pyrophosphate, 5 mM MgCl2, 5 mM NaF, 100 mM NaCl, 0.1 µM okadaic acid, phosphatase inhibitor cocktail (Calbiochem) and Roche protease inhibitor cocktail (pH 7.5)] with dounce homogenizer (on ice, 20 strokes), sonicated (on ice, 20 pulses, 40% duty cycle, output scale 4), and solubilized with 1% NP-40 (Calbiochem) (1% final). Samples were cleared by incubation (1 h) with 5 µg non-immune IgG, and then with Dynabeads protein G (Invitrogen, Cat# 100.04D) for 30 min before centrifugation (2,800 g, 4°C, 5 min). Cleared lysates from Hela cells (2 mg/IP) were incubated (overnight, 4°C) with mouse anti-Plk1 (10 µg/IP) (Millipore, Cat# 05-844) and non-immune mouse IgG (10 µg/IP), followed by incubation (1 h) with fresh Dynabeads protein G as described in methods. Immunoblot data indicated that Plk1 co-immunoprecipitated with PDE3A, PKA-C, PP2A and Cdc25C. n = 3 experiments. (E) Subcelluar localization of Plk1 and PDE3A in WT and PDE3A-/- oocytes. As described in methods, at the indicated times, cultured oocytes were collected, fixed, and assessed for immunofluorescence of PDE3A and Plk1. In freshly prepared WT oocytes, Plk1 was detected in the cytoplasm, with some localization at MTOCs. Within 1 hour, Plk1 migrated into the nucleus, and at GVBD, it was detected at MTOCs and kinetochores. In cultured WT oocytes PDE3A was partly co-localized with Plk1 at the spindle apparatus. In cultured PDE3A-/- oocytes, even after 4 hours, Plk1 immunofluorescence was only detected in the cytoplasm, with some localization at MTOCs, but not in the nucleus. No PDE3A signal was detected in PDE3A-/- oocytes. n = 3 experiments. Bar, 20 µm.](/cms/asset/0ddbd1c2-c1ea-481c-a26b-f49058ef6173/kccy_a_10914090_f0009.gif)
Figure 10 Proposed effects of PDE3A on regulation of cAMP/PKA signaling during meiotic progression in mice oocytes. In PDE3A-/- oocytes, activated PKA phosphorylates and inhibits Cdc25B and Plk1 directly, but phosphorylates/activates Wee1 kinase, and Myt1 is not subject to inhibition by Plk1. The integrated effect of these PKA-induced phosphorylations is inactivation of Cdc2 and maintainence of G2/M meiotic arrest. In WT oocytes, during GVBD, PKA is translocalized to the nucleus, where it may phosphorylate histone H3 to initiate DNA condensation; PKA-translocalization, histone H3 phosphorylation, and chromosome condensation does not occur in cultured PDE3A-/- oocytes. This scheme does not include possible “feed-forward amplification” of meiotic progression by activated MPF, i.e., activated Cdc2 activates Cdc25B and Plk1 (which inhibits Myt1), and inhibits Wee1 kinase, allowing further activation of MPF.
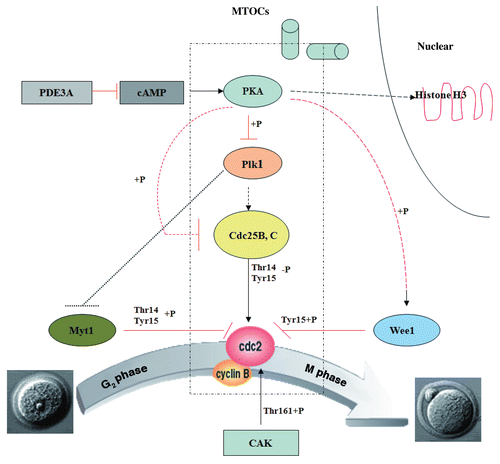
Table 1 Affymetrix microarray of key genes for G2/M transition in WT and PDE3A-/- oocytes