Figures & data
Figure 1. The extent of inhibition of TRPM7 channels by extracellular 2-APB depends on cytosolic pH buffering. Jurkat T cells were dialyzed with Mg2+-free internal solution containing 1 mM HEPES which led to activation of TRPM7 channels. (A) Monovalent TRPM7 current-voltage relations obtained from voltage ramps in the absence and presence of 100 µM 2-APB (100th voltage ramp after drug application). Reduction in current magnitude is voltage-independent. (B) Time course of development and inhibition of TRPM7 current in the same cell as in (A). Zero indicates the time of break-in. 2-APB was applied after ~10 min of dialysis with the Mg2+-free pipette solution containing 1 mM HEPES. Symbols correspond to measurements of TRPM7 current amplitude taken at -100 mV every 2.5 sec. 2-APB induced a slow reduction in current magnitude which was almost fully reversed upon washout of the compound. (C) Time course of TRPM7 current development with Mg2+-free internal solution containing 140 mM HEPES. 300 µM 2-APB was applied for the duration indicated by horizontal bar on top, followed by addition of TRPM7 channel blocker spermine (10 µM) in presence of 2-APB. Note that in this cell TRPM7 current was pre-activated at break-in.Citation26 (D) Inhibition of TRPM7 channels by 100 µM 2-APB is relieved by ammonium. 100 µM 2-APB was applied as indicated by the horizontal bar and elicited pronounced inhibition of TRPM7 current. Subsequent perfusion of 100 µM 2-APB + 50 mM NH4Cl resulted in recovery from inhibition. (E) Summary of experiments similar to those shown in (A–D) from multiple cells. Percent inhibition was calculated by dividing the current amplitude measured at ramp number 100 after the start of 2-APB application by the current amplitude measured immediately before. Each of bars in light colorshows the extent of current inhibition with 1 mM HEPES in the pipette and the indicated extracellular concentration of 2-APB and NH4+. Inclusion of 140 mM HEPES in the recording pipette (dark gray bars) prevented significant reductions of TRPM7 current amplitude. Numbers above the bars indicate the number of cells tested. Cells exposed to spermine were not included in the analysis. For cells treated with 2-APB+NH4+, the current amplitude at 70th ramp after NH4+ application was divided by current amplitude measured immediately before 2-APB application. Data represent mean ± SEM. Relevant significantly different pairs are shown by asterisk. *p < 0.001.
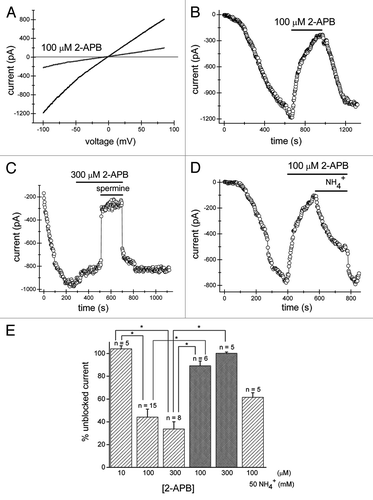
Figure 2. 2-APB inhibits TRPM7 channels from extracellular but not intracellular face of the plasma membrane. Recordings of TRPM7 currents were performed with 1 mM HEPES as described in the legend. (A) Monovalent TRPM7 current-voltage relations obtained in the absence and presence of external 100 µM 2-APB when the intracellular solution contained 100 µM 2-APB. (B) 100 µM 2-APB-containing pipette solution did not prevent normal activation of TRPM7 currents in Jurkat T cells. Addition of extracellular 2-APB at the same concentration inhibited the current as in .
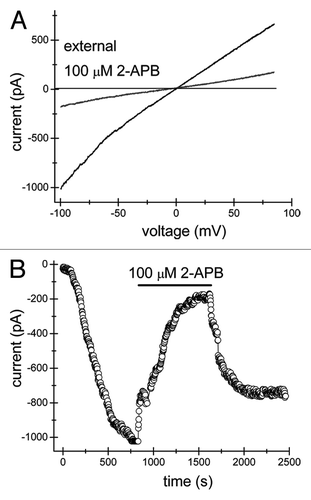
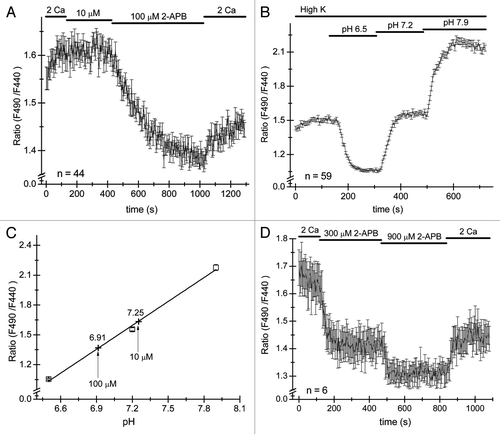
Figure 3. 2-APB reversibly acidifies Jurkat T cells. Cells were loaded with pH-sensitive dye BCECF-AM and ratiometric signals measured by alternatively illuminating the cells in the microscope field with 440 nm and 490 nm wavelengths every 12 sec and ratios of emitted light intensities computed. (A) 10 µM and 100 µM 2-APB was applied to Jurkat cells, followed by washout. 100 µM elicited a slow decrease in fluorescence ratios which was partially reversible. Measurements were taken after steady-state pH was achieved and plotted against the pH of the extracellular solution. Only responses from cells sensitive to 2-APB were analyzed. (B and C) Calibration experiment in cells on the same coverslip as used in (A). Cells were superfused with high K+ solution containing 10 µM nigericin (see Materials and Methods) at pH values of 6.5, 7.2 and 7.9. Calibration curve was constructed by plotting mean ratio values at steady-state in panel (B) against external pH. The data were fitted with a linear function. Ratio measurements from panel (A) are superimposed on the calibration curve as cross symbols. Note that the cells were initially incubated in high K+ solution that did not contain nigericin (B). (D) Application of 300 and 900 µM 2-APB acidifies the cytoplasm in a dose-dependent manner. The numbers of cells used to construct each graph is indicated in panels (A, B and C).