Figures & data
Figure 1. Multifunctional interactions of VGSCs. Basic topology of the pore-forming α subunit is shown, consisting of four homologous domains each containing six transmembrane segments. Segment four contains the voltage sensor.Citation6 The smaller β subunits contain an extracellular immunoglobulin (Ig) loop, transmembrane domain and an intracellular C-terminal domain.Citation88 β1 and β3 are non-covalently linked to the α subunit, whereas, β2 and β4 are covalently linked through disulfide bonds.Citation2 The alternative splice variant, β1B, lacks a transmembrane domain.Citation89 α subunits interact with a number of other signaling molecules, including p11,Citation90 protein kinase A (PKA),Citation91 protein kinase C (PKC),Citation92 ankyrin G,Citation93 MOG1,Citation94 fibroblast growth factor-homologous factor 1B (FHF1B),Citation95 calmodulin,Citation96 NEDD4,Citation97,Citation98 syntrophinCitation99 and dystrophin.Citation100 Several of the β subunits interact with other cell adhesion molecules and regulatory proteins, including tenascin C,Citation101 tenascin R,Citation101,Citation102 contactin,Citation103 N-cadherin,Citation104 neurofascin (NF)155,Citation105 NF186,Citation105 NrCAM,Citation105 ankyrin G,Citation104 receptor protein tyrosine phosphatase β (RPTPβ),Citation106 PKACitation107 and fyn kinase.Citation68 The β subunits are substrates for proteolytic cleavage by α-secretase, BACE1 and γ-secretase.Citation108,Citation109 The intracellular domain of β2 is proposed to regulate gene expression in the nucleus.Citation71 ψ, glycosylation sites. Figure was produced using Science Slides 2006 software.
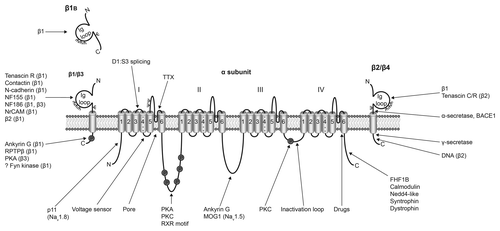
Table 1. VGSC subtype expression in cancer
Table 2. Metastatic cell behaviors regulated by VGSCs
Figure 2. α subunit involvement in pH-dependent cellular invasion. Na+ influx through Nav1.5 is proposed to activate the Na+/H+ exchanger NHE1, co-expressed with Nav1.5 in lipid rafts contained within the caveolae of invasive breast cancer cells.Citation59 Increased NHE1 activity results in increased H+ efflux, which, in turn, enhances proteolytic activity of cysteine cathepsins B and S, which degrade the extracellular matrix, permitting invasion.Citation52,Citation59 The mechanism by which Nav1.5 enhances NHE1 is not yet clear. Figure was produced using Science Slides 2006 software.
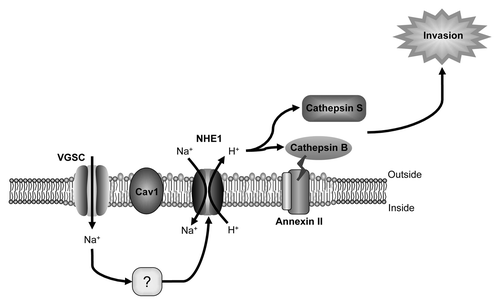
Figure 3. Nav1.5-regulated gene transcriptional network controlling invasion. Oval nodes represent regulatory genes, gray boxes represent effector genes and white boxes represent gene ontology categories. Arrows indicate activation and tees show repression. Reprinted by permission from the American Association for Cancer Research: House CD et al., Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res 2010; 70:6957-67; PMID:20651255.
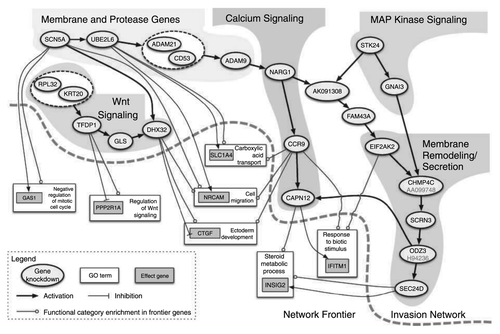
Figure 4. Neurite outgrowth and excitability regulated by β1 and Nav1.6. (A) β1-mediated neurite outgrowth is inhibited by the Scn8a null-mutation. Neurite lengths of wild-type and Scn8a-null cerebellar granule neurons grown on monolayers of control and b1-expressing fibroblasts. Data are mean and SEM ***p < 0.001. (B) Proposed model for Na+ current reciprocal involvement in β1-mediated neurite outgrowth. Complexes containing Nav1.6, β1 and contactin are present throughout the neuronal membrane in the soma, neurite and growth cone. Na+ influx is required for β1-mediated neurite extension and migration. VGSC complexes along the neurite participate in cell-cell adhesion and fasciculation. β1 is also required for Nav1.6 expression at the axon initial segment, and subsequent high-frequency action potential firing. Electrical activity may further promote β1-mediated neurite outgrowth at or near the growth cone. Figure reproduced with permission.Citation56