Figures & data
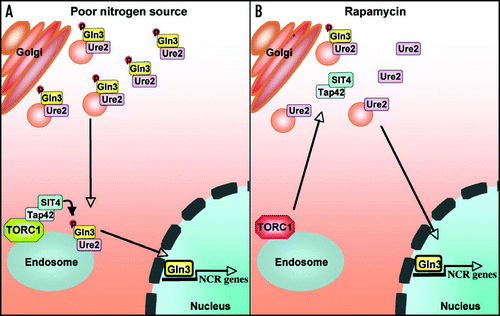
Figure 1 NCR gene expression in response to poor nitrogen source or rapamycin is governed by distinct signaling circuits. (A) Upon shift from a rich to a poor nitrogen source, free Gln3-Ure2 complexes associate with Golgi-derived vesicles to be transported to endosomes or a later compartment where Tap42-Sit4 complexes are membrane-tethered via TORC1. This trafficking step results in Gln3 dephosphorylation, Gln3-Ure2 complex dissociation, and Gln3 nuclear import. (B) Rapamycin-induced Gln3 nuclear translocation bypasses Golgi-to-endosome trafficking by liberating membrane-tethered Tap42-Sit4 complexes, which then dephosphorylate Gln3 in the cytosol or on Golgi-derived vesicles to enable nuclear import and activation of NCR gene expression.