Figures & data
Figure 1 Axonal actin patches and PIP3 microdomains. (A) Example of the development of an axonal actin patch along the axon of a cultured sensory neuron as revealed by time-lapse imaging of eYFP-β-actin. The numbers in the panels reflect seconds elapsed. At 0 sec, the patch becomes detectable as a localized accumulation of eYFP-actin (arrowhead) amongst the dim background signal of the axon. Over the next 24 sec the patch grows in intensity. Between 12–18 sec the patch gives rise to a filopodium (arrow). (B) Example of the formation and development of an axonal microdomain of PIP3 accumulation as revealed by PH-GFP imaging. The microdomain becomes detectable above background at 6 sec (arrowhead) and by 24 sec it has almost returned to background levels. (A and B) are from different time-lapse sequences. Dual-channel imaging of mCherry-actin and PH-GFP in the same axon is reported elsewhere in reference Citation10. (C) Schematic summarizing the effects of NGF on the number of axonal actin patches and filopodia. The axon is shown as a black bar. NGF treatment (Post NGF) doubles the numbers of axonal actin patches and filopodia. However, the percentage of patches that give rise to a filopodium is not affected by NGF.
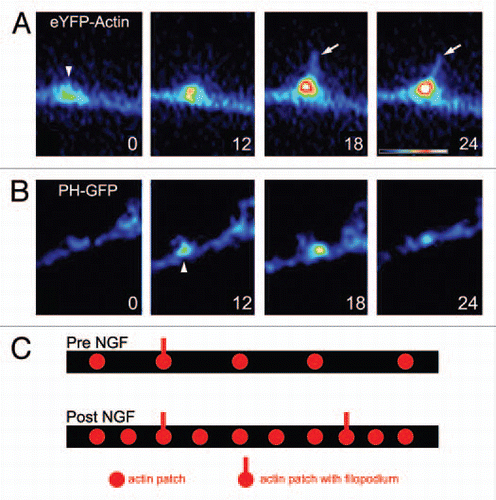
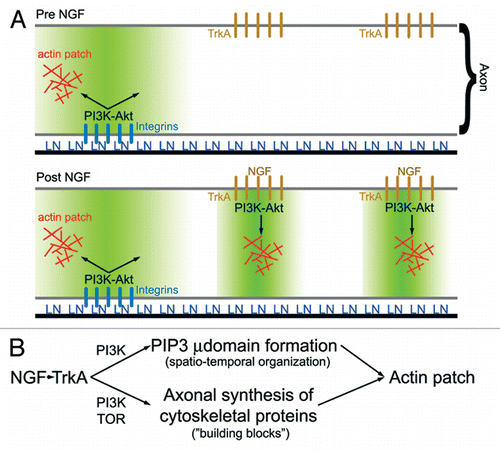
Figure 2 (A) Schematic summarizing the role of PI3K signaling in laminin (LN) and NGF mediated formation of axonal actin patches.Citation10 In the absence of NGF treatment (top part), LN signaling through integrins promotes the formation of PI3K-dependent PIP3 axonal microdomains (region shaded green). Axonal actin patches form in the vicinity, of integrin clusters. Following NGF treatment, TrkA signaling induces formation of additional PI3K-dependent PIP3 axonal microdomains, and actin patch formation colocalizes with clusters of TrkA receptors. Thus, localized PIP3 microdomains act to orchestrate the focal assembly of actin patches at discrete foci along the axon. (B) Schematic summarizing the hypothesis that NGF-induced intra-axonal protein synthesis of cytoskeletal proteins involved in the formation of actin patches is required to maintain a balance between the increase in the number of PIP3 microdomains (µ domains) and the availability of proteins utilized by microdomains to drive formation of actin patches. PIP3 microdomains regulate the spatio-temporal aspects of actin patch initiation and development along axons, while increases in intra-axonal protein synthesis provide the molecular “building blocks” required for the coordinated assembly of actin patches by PIP3 microdomains. Inhibition of intra-axonal protein synthesis induced by NGF is predicted to have no effect on the formation of PIP3 microdomains while blocking the ability of the NGF-induced microdomains to assemble actin patches due to insufficient supply of cytoskeletal proteins.