Figures & data
Figure 1 (A) Two views of the solution NMR structure of MinE from Neisseria gonorrhoeae (PDB accession number 2KXO), with a model of the more open conformation suggested by NMR dynamics data on the right hand side. Each subunit in the dimer is shown in a different colour, and β-strands β1, β2 and β3 labeled for each subunit. Sidechain atoms for residues that directly participate in MinD interactions are also shown, with the inaccessible Leu-22 highlighted in red and the partially inaccessible residues (Arg-21 Ile-25) shown in orange. (B) The solution NMR structure of residues 31–88 from E. coli MinE (1EV0), showing the different dimer interface formed by residues corresponding to β3 in the full-length structure.
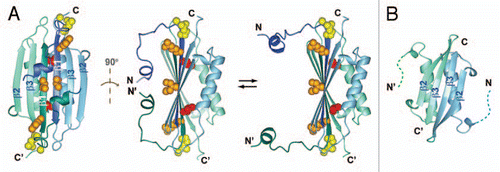
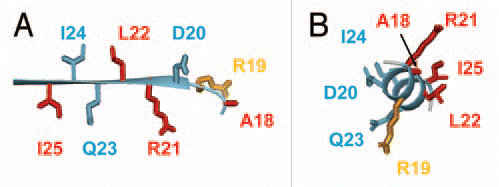
Figure 2 Magnified view of the region of MinE containing residues that bind to MinD, either in (A) the β1 structure as it appears in the dimer or (B) in a hypothetical α-helical structure. Sidechains that are critical for MinD binding are shown in red, those that play a smaller role in the interaction in orange and those that can be mutated and still give rise to wild-type function are in blue.