Figures & data
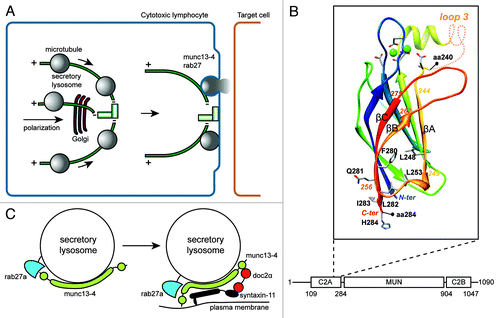
Figure 1. Release mechanism of SL in CTL. (A) Antigen-dependent recognition of a target cell induces formation of a immune synapse between the two cell types. The CTL rapidly reorganizes its MTOC and the microtubule network toward this contact site. SL move in a minus-end direction along microtubules and cluster at the MTOC. Docking of the MTOC at the plasma membrane positions SL for fusion and lytic content release at the immune synapse. (B) Model of the 3D structure of the C2A domain of munc13–4 (aa 111–284). The model is based on the alignment of the C2A domain of human munc13–4 with the C2B domain of rat munc13–1, whose structure has been solved.Citation3 The limits of the 240–284 segment, including βA, βB, βC, are shown, as well as amino acids discussed in the text. Loops which were not modeled are represented by dashed lines. Amino acids of the Ca2+-binding site are shown, together with the bound Ca2+ ions (green spheres). (C) Hypothetical model for the function of munc13–4 rab27a complex. Immune signaling brings munc13–4 and rab27a in each other's vicinity. Colocalization facilitates the interaction between the two proteins and binding of rab27a could fold munc13–4 in a conformation that bridges SL membrane and plasma membrane.Citation4 The complex can also organize interactions with other proteins important for secretory lysosome release like syntaxin-11 and doc2α which then interacts with SNAP-23, establishing a regulatory circuit for controlling a cognate SNARE complex.