Figures & data
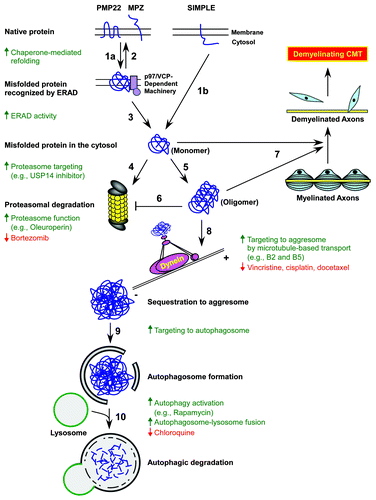
Figure 1. Protein quality control systems are potential targets for mechanism-based treatments of demyelinating Charcot-Marie-Tooth disease (CMT). Genetic mutations and increased protein expression levels linked to demyelinating CMT induce misfolding of hydrophobic proteins such as peripheral myelin protein 22 (PMP22), myelin protein zero (MPZ), and SIMPLE in Schwann cells (step 1a and 1b). Chaperones at the endoplasmic reticulum (ER) and the cytosol refold misfolded PMP22 and MPZ proteins (step 2), but when refolding is not possible, these proteins are recognized and retrotranslocated to the cytosol by the p97/VCP-dependent ER-associated degradation (ERAD) machinery (step 3). In contrast, misfolded SIMPLE is translocated to the cytosol by an ERAD-independent mechanism (step 1b). These misfolded proteins are targeted to the 26S proteasome by K48-linked poly-ubiquitination for degradation (step 4). When the chaperone and proteasome systems are damaged or overwhelmed, misfolded proteins accumulate and aggregate into toxic oligomers (step 5) that could inhibit proteasome function (step 6) and impair myelination by Schwann cells, leading to demyelinating CMT (step 7). The aggresome-autophagy pathway is another protein quality control system in which misfolded and aggregated proteins are transported by the microtubule-dependent dynein motor complex to the aggresomes (step 8). Aggresomes not only sequester toxic misfolded and aggregated proteins but also recruit autophagic membrane for the formation of autophagosomes (step 9) and subsequent degradation by lysosomal hydrolases upon autophagosome-lysosome fusion (step 10). Therapeutic strategies and currently available agents that enhance the targeting of misfolded and aggregated proteins for turnover are indicated by the color green (upward arrows), and drugs exacerbating the CMT neuropathy phenotype are marked by the color red (downward arrows).