Figures & data
Figure 1. Astroglial gap junctional communication prevents amplification of neuronal activity and reduces neuronal network activity. Synchronous recordings of hippocampal CA1 extracellular field excitatory postsynaptic potentials (fEPSP) (A) and astroglial membrane depolarizations (C) were performed and representative traces are illustrated. (B) Increasing the stimulation length from 0.1 to 0.5 ms resulted in comparable presynaptic activity (fiber volley), but enhanced postsynaptic activity (charge transfer, p < 0.05) in Cx30−/−Cx43−/− mice (n = 6), as compared with the wildtype mice (n = 7). Scale bar 0.2 mV, 25 ms. (C, D) The extracellular potassium accumulation is increased (amplitude p < 0.05) and prolonged (decay, p < 0.001 for 0.1ms pulse width and p < 0.005 for 0.5 ms pulse width) in slices from Cx30−/−Cx43−/− mice (n = 6) compared with wildtype mice (n = 7), as measured by the astroglial membrane depolarization. Scale bar, 1 mV, 1 sec. (E, F) Spontaneous bursts, induced by inhibition of GABAergic transmission (100 µM picrotoxin) and removal of extracellular Mg2+, and recorded extracellularly in the hippocampal CA1 area, occur more frequently in Cx30−/−Cx43−/− mice (p < 0.001, n = 16) than in wildtype mice (n = 16). Scale bar, 0.2 mV, 30 sec. However, amplitude (p < 0.001), as well as charge transfer (p < 0.001), are reduced in Cx30−/−Cx43−/− mice (n = 16, WT n = 16). High magnifications of representative traces are shown on the right. Scale bar, 0.2 mV, 1s.
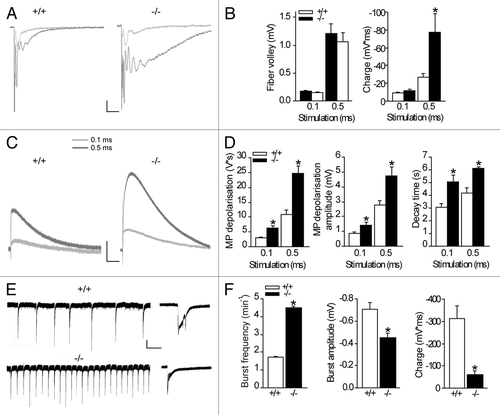
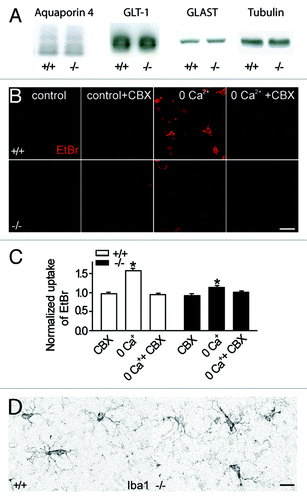
Figure 2. Absence of compensatory upregulation of water channels, glutamate transporters, opening of pannexin hemichannels or reactive microglia in Cx30-/-Cx43-/- mice. (A) Quantitative immunoblot analysis of hippocampal extracts from Cx30-/-Cx43-/- mice (n = 3) and wild type (n = 3) revealed no difference in protein expression for the water channel aquaporin 4 (~30 kD) and the glial glutamate transporters GLT-1 (~70 kD) and GLAST (~60 kD). Tubulin was used as a loading control. (B, C) Under control conditions, no enhanced ethidium bromide (EtBr) uptake was detectable in astrocytes from Cx30-/-Cx43-/- mice. Preincubation with carbenoxolone (CBX, 200 µM) for 15 min, a connexin and pannexin hemichannel blocker, has no effect on basal astroglial ethidium bromide uptake in wildtype (n = 50) and Cx30-/-Cx43-/- mice (n = 30). Removal of extracellular calcium (0 Ca2+) induced a carbenoxolone sensitive EtBr uptake in astrocytes from wildtype mice (n = 50), and to a much smaller extend in Cx30-/-Cx43-/- mice (n = 36). Scale bar, 25 µm. (D) Immunostaining of hippocampal slices with the microglia marker Iba-1 revealed no sign of microglia activation in slices from Cx30-/-Cx43-/- mice (n = 3; wild type n = 3). Scale bar, 10 µm.