Figures & data
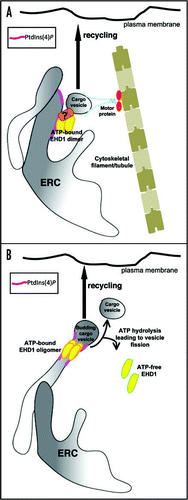
Figure 1 Potential models depicting mechanisms of EHD1-mediated recycling from the ERC. (A) EHD1 dimers in their ATP-bound state are recruited to the PtdIns(4)P-enriched tubular membranes of the ERC. EHD1 can then either directly or indirectly associate with cargo vesicles (via an unknown protein(s) such as a Rab effector) coming from the early endosome and provide a platform for association of motor proteins that might drive recycling vesicles back to the plasma membrane. (B) A second proposed mechanism for EHD1-mediated recycling is that ATP-bound EHD1 associates with tubular PtdIns(4)P-enriched membranes. Once on these structures, EHD1 utilizes its intrinsic ATPase activity and acts as a ‘pinchase’ to promote membrane fission of the vesicles budding from the ERC tubules. These pinched-off cargo vesicles can then be carried back to the plasma membrane by the motor proteins as described above.