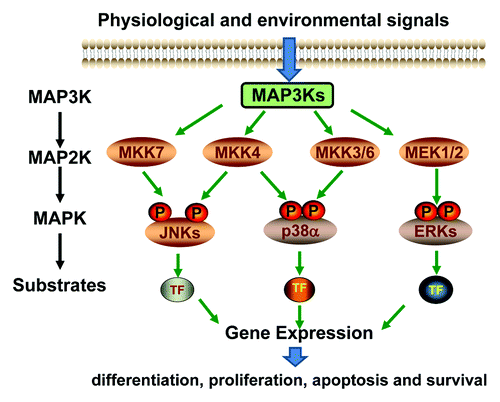
Figures & data
Figure 2. Schematic diagrams of embryonic stem cell differentiation. (A) Diagrammatic illustration of the early stage of fetal development and the in vivo origin of embryonic stem cells. (B) In vitro ESC differentiation using the “hanging drop” protocol. Differentiation is initiated when the cells are grown in the absence of anti-differentiation factors, such as LIF and fibroblast feeder cells. Approximately 500 cells/20 µl were applied to the lids of tissue culture plate, as hanging drops, to force the cells aggregate to form embryoid bodies (EBs). The EBs can further differentiate in vitro, leading to the generation of cell types of ectoderm, endoderm and mesoderm lineages.
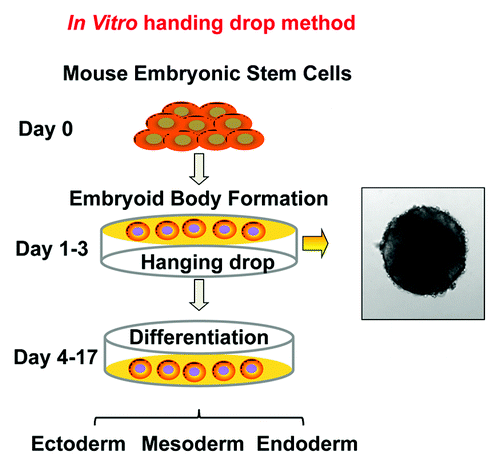
Figure 3. The differentiation profiles of EB. Wild type mouse ESCs were subjected to in vitro differentiation, following the “hanging drop” protocol and the EBs were transferred to a 10 cm2 bacterial plate at approximately 100 EBs per plate. Under this condition, the ESCs spontaneously differentiate into mesoderm, ectoderm and endoderm lineages. RNA was isolated at day 13 of differentiation and lineage specific markers were examined by real time RT-PCR. The most abundantly expressed genes in the EB culture were those for the mesodermal lineage, including genes specific for primitive mesoderm, Fibroblast growth factor 5 (Fgf5), Brachyury and Nodal, for chondrocytes, Collagen 2a2 (Col2a2) and Metalloproteinases 2 (Mmp2), and for myocytes, Myosin light chain (Mlc) and Myosin heavy chain a (Mhca) and Mhcb. Less abundantly expressed were genes for the endodermal lineage, including markers for definitive endoderm Cytokeratin 19 (Ck19) and liver endoderm, such as Forkhead box A2 (Foxa2) and GATA binding protein 4 (Gata4). The least expressed were genes for the ectodermal lineage, including markers for epidermis, such as Fillagrin, Loricrin and Involucrin, for neuronal lineages, such as Notch-1, Tubulin β 3 (Tubb3) and Vimentin. The sequences of forward primers used in PCR are: Gapdh, 5′-AACGACCCCTTC ATTGACC-3′, Ck19, 5′-TCAATGATCGTCTCGCCTCCTACT-3′, Foxa2, 5′-AAGTATGCTGG GAGCCGGAAGAT-3′, Gata4, 5′-CTG TCA TCT CAC TAT GGG CAC-3′, Filaggrin, 5′-GAAACGATATACCTG GAGATGC3′, Loricrin, 5′TGAGGAGACACTAGAATTGGG3′, Involucrin, 5′-GAGAAGCAGCATCAGAAGCC-3′, Notch-1, 5′-TCTGCTTATGCCTCAAGGGAACCA-3′, Tubb3, 5′-TTCTGGTGGACTTGGAACCTG GAA-3′, Vimentin, 5′-CTTTACTCAACTTTCCAGAGCC-3′, Fgf5, 5′-AGAGTGGGCATCGGTTTC-3′, Brachyury, 5′-TGCTGCAGTCCCATGATAACTGGT-3′, Nodal 5′-CCAACCATGCCTACATCCAG-3′, Col2a1, 5′-ATCTGCACTGAATGGCTGACCTGA-3′, Mmp2, 5′- AACCAGCCTTCTCCTTCAC-3′, Mlc, 5′- CTCCAAGAACAAGGACACTG-3′, Mhca, 5′-CTGCTGGAGAGGTTATTCCTC G-3′, Mhcb, 5′-TGCAAAGGCTCCAGGTCTGAGGGC-3′
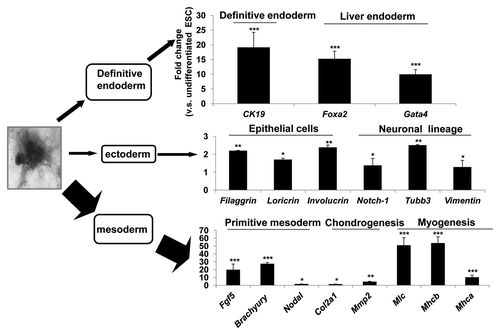
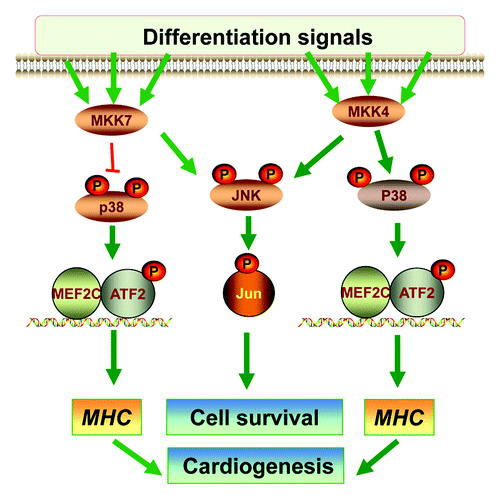
Figure 4. The roles of MKK4 and MKK7 in differentiation. A Schematic diagram summarizes the roles of MKK4 and MKK7 in vitro ESC differentiation. The MKK4 and MKK7 have complementary roles in activation of the JNK-c-Jun cascades that may be required for differentiated cell survival. Hence, loss of both MKK4 and MKK7 leads to senescence of the differentiated cells. On the other hand, MKK4 is essential for activation of the p38-ATF2/MEF2C cascades, while MKK7 may attenuate p38 activation. Consequently, the Mkk4(-/-) ESCs are defective in myosin heavy chain (MHC) induction and cardiomyocyte differentiation, but Mkk7(-/-) ESCs have enhanced MHC expression and cardiogenesis.