Figures & data
Figure 1. F-actin dynamics and dendritic spine formation. (Step I) Dendrite first extends filopodia to explore the environment. The F-actin bundle is the component of the cytoskeleton that supports the structure of filopodia. (Step II) Once filopodia make contact with the presynaptic button and initiates the synaptic interaction, F-actin cytoskeletons undergo remodeling by depolymerization and severing. Filopodia will then withdraw and transform to mushroom-like dendritic spines. Without synaptic contact, filopodia will withdraw and disappear later. Since axonal contact with dendritic filopodia induces calcium influx of dendritic filopodia, it is very likely that calcium triggers the transformation of filopodia to dendritic spines. (Step III) To enlarge the dendritic spine, F-actin cytoskeletons increase the branching level. Therefore, molecules promoting F-actin branching are expected to play a role in regulation of spine morphology or density.
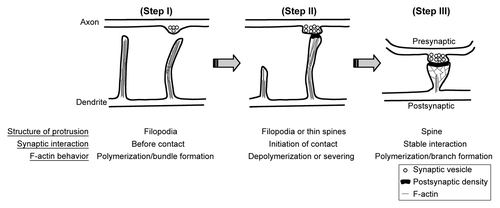
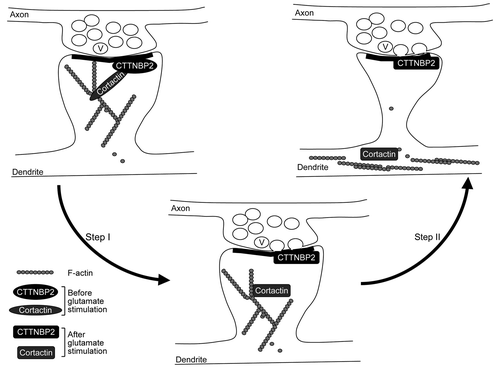
Figure 2. Coordination of neuronal activity and CTTNBP2-dependent F-actin remodeling. Cortactin binds both F-actin and Arp2/3 and thus stabilizes F-actin polymers and branching; CTTNBP2 stably resides in the dendritic spines and may anchor the cortactin-F-actin cytoskeleton in the dendritic spine. The interaction of cortactin and CTTNBP2 regulates dendritic spine formation. Additionally, when postsynaptic glutamate receptors are activated by glutamate released from the presynaptic button, the signal may then posttranslationally modify CTTNBP2 and/or cortactin and result in dissociation of CTTNBP2 and cortactin (Step I). Consequently, cortactin and F-actin will then move to the dendritic shaft (Step II). The process is expected to regulate remodeling of dendritic spines upon synaptic stimulation. V, synaptic vesicle.