Figures & data
Figure 1. Applying local mechanical forces to living NIH3T3 cells with simultaneous AFM and LSCM. (A) An NIH3T3 cells transiently expressing actin-EGFP. The AFM cantilever is positioned above the cell nucleus and used to apply local nanonewton forces. (B) Heat map lateral actin deformation in a cell exposed to a 20nN stimulus at the position marked with an ‘x’. Highly localized deformations take place far form the point of contact and evolve in time. Experimental details in reference Citation11. Figure adapted from reference Citation11. Scale bars in both (A) and (B) are 15 μm.
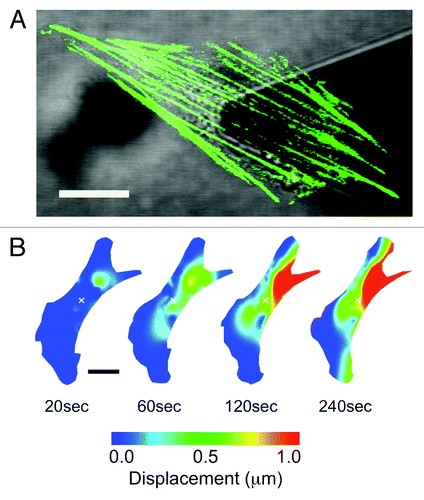
Figure 2. Quantifying strain dynamics in actin stress fibers. (A) Actin-EGFP stress fibers photobleached every 5 μm to create a segmented appearance (stress fibers do remain intact). As the stress fibers undergo stretch and contraction the segments will change length allowing the quantification of internal strain. (B) Strain dynamics taking place along stress fibers are heterogeneous and evolve in time. Negative strain values correspond to regions undergoing contraction and positive strain values correspond to regions undergoing stretch. Experimental details in reference Citation11. Figure adapted from reference Citation11. Scale bars in both (A) and (B) are 15 μm.
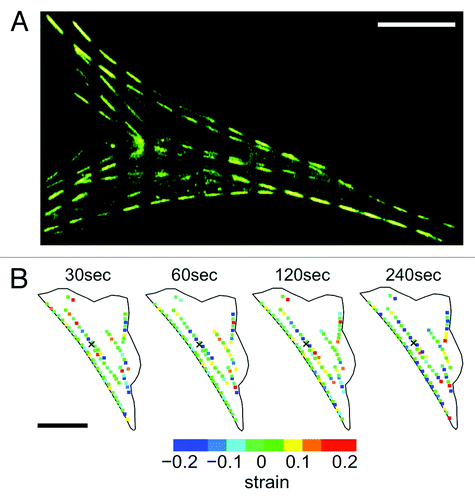
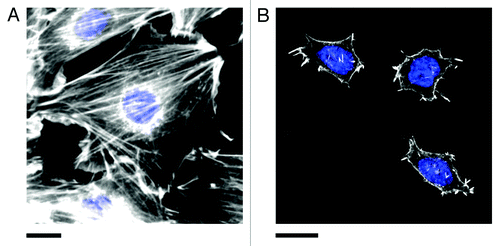
Figure 3. Actin stress fibers in (A) NIH3T3 and (B) pluripotent ES-D3 mouse embryonic stem cells (mESCs). Cells were fixed and stained for actin (phalloidin conjugated to Alexa Fluor 546, white) and the nucleus (DAPI, blue). Stress fibers are numerous and highly organized in committed NIH3T3 cells. Pluripotent mESCs (confirmed by staining for the presence of the transcription factor OCT4, not shown) are generally significantly smaller than NIH3T3 cells and display fewer stress fibers. The actin is generally diffuse and poorly organized. However, small punctate actin protrusions are observed. Experimental details in reference Citation11. Scale bars in both (A) and (B) are 20 μm.