Figures & data
Figure 1. (A) Structure of the talin rod domain consisting of 12 helices (upper panel). The rod domain which is unfolded under forceCitation28 (lower panel) is shown by the black arrows in the upper panel. (B) Schematic drawing of the experimental setup to apply force to a single actin filament.Citation23 One end of an actin filament (red) was tethered to a bead fixed on a coverslip, and the other end of the filament was tethered to a small bead manipulated by optical tweezers. The lower actin filament is not tensed, and is severed by cofilin. (C) (i), Schematic drawing of the experimental setup to trace the torsional fluctuations of a single actin filament. An actin filament is tethered on the coverslip via gelsolin, and a bead was attached to the lower end of the actin filament. Rotation of the bead is monitored using fluorescent small beads that are attached on the large bead. (ii), Rotational angular fluctuations of a bead attached to an actin filament during the time the large bead was trapped, but not stretched, by optical tweezers. (iii). The rotational angular fluctuations were decreased when the actin filament was stretched by moving the trapping point in downward direction. The data show the results at zero and ca. 5 pN stretch force; one can change the applied force by increasing the laser power of the optical tweezers. Panels A and C are based on studies (28) and (23), respectively.
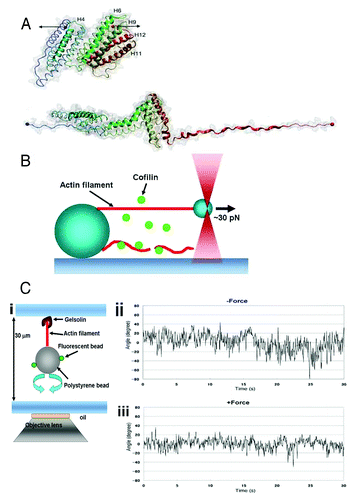
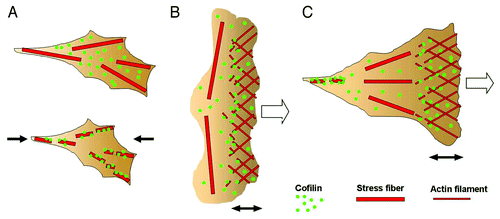
Figure 2. (A) When a certain amount of tension was generated in the stress fibers in adherent cells, the binding of cofilin to the stress fibers was reduced (upper panel). When the tension was reduced by relaxing the cell substratum (the direction is indicated by the black arrows), cofilin bound and started to disassemble the stress fibers (lower panel). (B) Schematic drawing of the actin cytoskeleton in a locomoting keratocyte. The actin filaments are disassembled by cofilin near the leading edge of the cell. The prominent transverse stress fibers generating a large amount of contractile force are not disassembled. (C) Schematic drawing of the actin cytoskeleton in an adherent cell during migration. The actin filaments are disassembled in the trailing region of the cell, where the tension in the stress fibers is low, while the stress fibers generating tension in the middle region of the cell are not disassembled. The open arrows in B and C denote the direction of cell migration. The double-headed arrows indicate the width of the lamellipodia.