Figures & data
Figure 1. western blots demonstrating the effects of c-Cbl depletion on ∆F508-CFTR abundance in whole cell lysates (WCL) at steady-state in polarized CFBE41o- cells. Cells were transfected with 50 nM siRNA against the human c-Cbl gene (s-ic-Cbl) or the non-silencing negative siRNA control (si-CTRL). Low temperature (27°C) for 48h was used to rescue the biosynthetic processing defect of ∆F508-CFTR. Representative western blots (A, the bottom panel) and summary of experiments (B) demonstrating that si-c-Cbl decreased the c-Cbl protein abundance in WCL. Low temperature (27°C) did not affect the c-Cbl silencing efficiency. Low temperature rescued the abundance of the mature, plasma membrane associated, fully glycosylated ∆F508-CFTR band C. Partial silencing of c-Cbl increased the steady-state abundance of ∆F508-CFTR band C in polarized CFBE41o- cells (A; the top panel and C). By contrast, partial silencing of c-Cbl did not increase the steady-state abundance of the partially glycosylated ∆F508-CFTR band B (A and C). Silencing c-Cbl in cells not subjected to low temperature (37°C) did not increase the abundance of the ∆F508-CFTR band C, indicating that c-Cbl does not facilitate the biosynthetic processing of ∆F508-CFTR (A). The ∆F508-CFTR abundance was normalized for actin. Actin expression was used as a loading control. *, p < 0.05 vs. siCTRL. Five experiments/group. Error bars, SE.
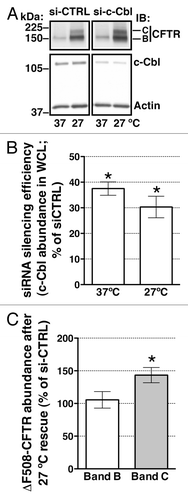
Figure 2. western blots demonstrating the effects of c-Cbl depletion on ∆F508-CFTR abundance in whole cell lysates (WCL) in polarized CFBE41o- cells as a function of time. Cells were transfected with 50 nM siRNA against the human c-Cbl gene (si-c-Cbl) or the non-silencing negative siRNA control (si-CTRL). Low temperature (27°C) for 48h was used to facilitate the biosynthetic processing of ∆F508-CFTR band C. Disappearance of ∆F508-CFTR band C (A and C) or band B (A and D) from WCL was monitored over time in the presence of 20 µg/ml cycloheximide (CHX) at 37°C (A, C and D) or 27°C (B–D). In si-CTRL-transfected cells, at least 50% of ∆F508-CFTR band C disappeared in 4h. Thus, data are reported at the 4h time point (C and D). Representative western blots (A) and summary of experiments (C) demonstrating that partial silencing of c-Cbl attenuated the disappearance of the temperature rescued ∆F508-CFTR band C at 37°C without affecting the stability of the Epidermal Growth Factor Receptor (EGFR) or Transferrin Receptor (TfR). Low temperature (27°C) alone decreased the disappearance of the rescued ∆F508-CFTR band C and partial silencing of c-Cbl had a synergistic effect and completely prevented the disappearance of the temperature rescued ∆F508-CFTR band C (B and C). Partial silencing of c-Cbl did not attenuate the disappearance of the ∆F508-CFTR band B at 37°C or 27°C (A, B and D). The ∆F508-CFTR abundance was normalized for ezrin. Ezrin expression was used as a loading control. *, p < 0.05 vs. time zero in siCTRL. Five experiments/group. Error bars, SE.
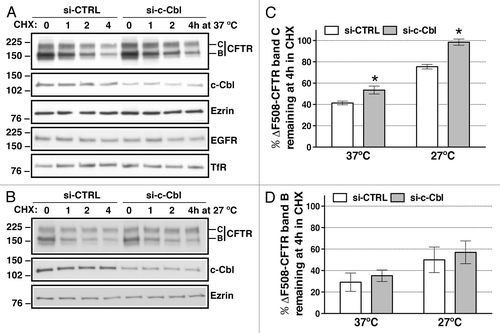
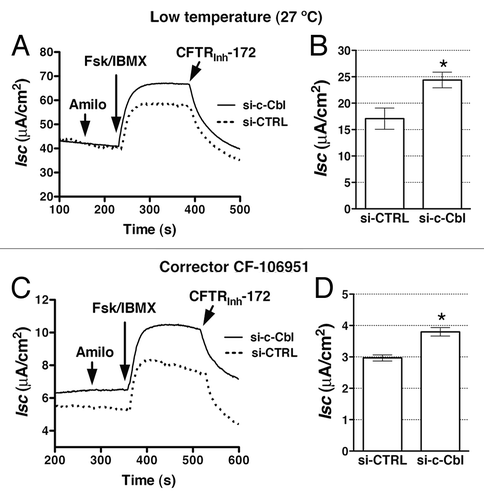
Figure 3. Ussing chamber experiments performed to determine the effects of c-Cbl depletion on the ∆F508-CFTR mediated Cl- secretion across CFBE41o- monolayers. Cells were transfected with 50 nM siRNA against the human c-Cbl gene (si-c-Cbl) or the non-silencing negative siRNA control (si-CTRL). Low temperature (27°C; A and B) or a corrector CF-106951 (C and D) for 48h was used to facilitate the biosynthetic processing and rescue abundance of ∆F508-CFTR band C. CFBE41o- cells were bathed in solutions with apical-to-basolateral Cl- gradient in the presence of amiloride (50 μM) in the apical bath solution to inhibit Na+ absorption through ENaC. Isc was stimulated with forskolin (20 μM) and IBMX (50 μM) added to the apical and basolateral bath solution. Thiazolidonone CFTRinh-172 (5 μM) was added to the apical bath solution to inhibit CFTR-mediated Isc. Data are expressed as net stimulated Isc, calculated by subtracting the baseline Isc from the peak stimulated Isc. Si-CTRL did not affect the forskolin/IBMX-stimulated Isc across CFBE41o- cells compared with the non-transfected cells (data not shown). Si-c-Cbl did not change the transepithelial resistance across cell monolayers (398.8 ± 72 Ωcm2 vs. 404.7 ± 140.8 Ωcm2 for the si-CTRL and si-c-Cbl transfected cells, respectively). Representative experiment (A) and summary of data (B) demonstrating that partial silencing of c-Cbl increased the forskolin/IBMX-stimulated Isc across CFBE41o-cells after the low temperature rescue. Moreover partial silencing of c-Cbl increased the forskolin/IBMX-stimulated Isc across CFBE41o-cells after the CF-106951 rescue (C and D). *, p < 0.05 vs. siCTRL. Six monolayers/group. Error bars, SE.