Figures & data
Figure 1. Establishing the LR axis is a 3-step process. In the first step, symmetry is broken; after the anterior-posterior and dorsal-ventral axes are established in the radially symmetrical egg, the LR axis can be defined. In the second step, the early asymmetries are translated to asymmetric expression of genes such as the Nodal-Lefty-Pitx2 cassette. Finally, this asymmetric gene expression is translated into the asymmetric positioning and shape of the internal organs.
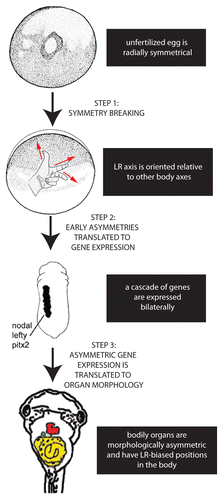
Figure 2. The role of ion flux in the intracellular model of LR asymmetry. (A) A chiral cytoskeleton asymmetrically distributes mRNAs that encode a number of ion transporters including H+ pumps and K+ channels. (B) The biased expression of these ion transporters in the ventral right blastomere establishes a LR difference in resting potential between the L and R sides (due to the flow of positive ions from the ventral right side of the embryo, the blastomeres in this part of the embryo become relatively negative). (C) With the exception of the ventral-most blastomeres, all of the blastomeres of the embryo are connected via a series of open gap junctions. The relatively negative nature of the ventral right blastomere establishes a net flow of positively charged signaling molecules such as serotonin to this portion of the embryo.Citation192-Citation195 While this scheme illustrating the amplification of cell chirality into multi-cellular gradients has best been worked out in Xenopus, it remains to be tested in other model species to determine how similar physiological pathways could map onto phyla with different bodyplans.
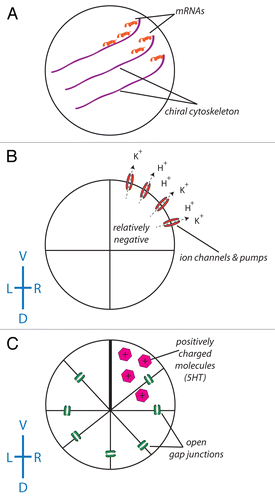
Figure 3. Centrosomes are implicated in 3 major models of LR asymmetry. (A) The centrosome itself is asymmetric. The centrosome has 2 centrioles (shown as red barrels) that are asymmetric in size; these are always found at a right angle to each other. The two centrioles and a matrix of proteins including γ-tubulin (orange lines) together form the MTOC. (B) In the asymmetric chromatid segregation model, the MTOC controls chromatid movements during cell divisions. (C) In the ion flux model, the MTOC serves to establish the chiral cytoskeleton, which eventually leads to the asymmetric distribution of ion transporters. (D) In the cilia model, the basal body sits at the base of the cilia and, through the movements of the cilia, generates asymmetric fluid flow.
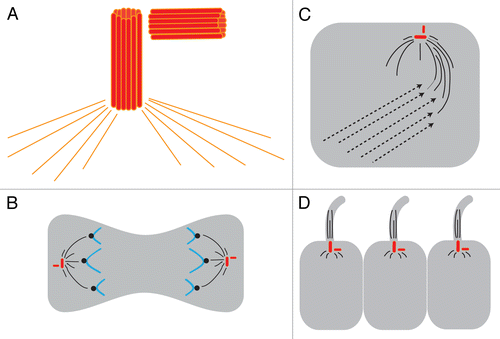
Figure 4. The centrosome could be the F-molecule orienting the 3 axes at fertilization. (A) The unfertilized egg, seen from the animal pole, is radially symmetric about the animal-vegetal axis but contains a chiral actin cytoskeleton.Citation47 (B) At fertilization, the sperm entry point produces the first “point” around the circumference that is different from any other. The direction of the actin cytoskeleton at this point establishes a linear LR axis—a convergence of the 3 axes that could enable the MTOC to be uniquely oriented and thus establish directional asymmetries in the microtubule cytoskeleton that would underlie asymmetric transport of intracellular components.Citation70

Table 1. Cytoskeletal proteins have a conserved role in LR patterning
Figure 5. Using experimental strategies to probe the early roles of cytoskeletal proteins on LR patterning. (A) Using strategy 1 (early vs. late injections), we found that expression of mutant TBCB mRNA altered LR patterning only when injected at 1-cell, indicative of a very early role for this tubulin binding protein. (B) Using strategy 2 (targeting to the GRP via the dorsal left [DL] blastomere vs. targeting to a region that does not contribute to flow at the GRP via the ventral right [VR] blastomere), we determined that expression of dominant negative CDC42 was effective at disrupting LR patterning, regardless of where it was targeted. These results indicate a GRP-independent role of CDC42 on orientation of the LR axis. (C) In contrast, expression of 5HT-R3A mRNA only disrupted LR patterning when targeted to the ventral right (VR) blastomere, signaling a GRP-independent role for 5HT. These results were previously reported in reference 154. (D) Using strategy 1, we found that early injections of mutant Kif3B mRNA effectively disrupted LR patterning. Using strategy 2, we observed that injections in the dorsal left (DL) blastomere were significantly more effective than injections in the ventral right (VR) blastomere, indicative of a role of Kif3B at the GRP. (E) To further probe the early role of Kif3B, we used strategy 3, injecting 1-cell embryos in a biased manner with the mutant Kif3B construct and a lineage label. When we post-sorted the tadpoles and scored them for heterotaxia, we observed a significant number with LR defects, regardless of whether the injections included the GRP (DL) or not (VR). These results suggest that Kif3B has an early role in LR patterning that is distinct from ciliary flow at the GRP, and likely has a second role in LR patterning at the GRP. For all panels, * indicates significant differences from controls (P < 0.01) and # identifies significant differences between the indicated groups.
![Figure 5. Using experimental strategies to probe the early roles of cytoskeletal proteins on LR patterning. (A) Using strategy 1 (early vs. late injections), we found that expression of mutant TBCB mRNA altered LR patterning only when injected at 1-cell, indicative of a very early role for this tubulin binding protein. (B) Using strategy 2 (targeting to the GRP via the dorsal left [DL] blastomere vs. targeting to a region that does not contribute to flow at the GRP via the ventral right [VR] blastomere), we determined that expression of dominant negative CDC42 was effective at disrupting LR patterning, regardless of where it was targeted. These results indicate a GRP-independent role of CDC42 on orientation of the LR axis. (C) In contrast, expression of 5HT-R3A mRNA only disrupted LR patterning when targeted to the ventral right (VR) blastomere, signaling a GRP-independent role for 5HT. These results were previously reported in reference 154. (D) Using strategy 1, we found that early injections of mutant Kif3B mRNA effectively disrupted LR patterning. Using strategy 2, we observed that injections in the dorsal left (DL) blastomere were significantly more effective than injections in the ventral right (VR) blastomere, indicative of a role of Kif3B at the GRP. (E) To further probe the early role of Kif3B, we used strategy 3, injecting 1-cell embryos in a biased manner with the mutant Kif3B construct and a lineage label. When we post-sorted the tadpoles and scored them for heterotaxia, we observed a significant number with LR defects, regardless of whether the injections included the GRP (DL) or not (VR). These results suggest that Kif3B has an early role in LR patterning that is distinct from ciliary flow at the GRP, and likely has a second role in LR patterning at the GRP. For all panels, * indicates significant differences from controls (P < 0.01) and # identifies significant differences between the indicated groups.](/cms/asset/e783c160-9043-4722-a179-74d8b282ceb2/kcib_a_10927155_f0005.gif)